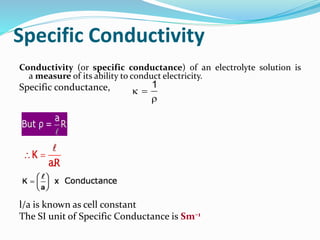
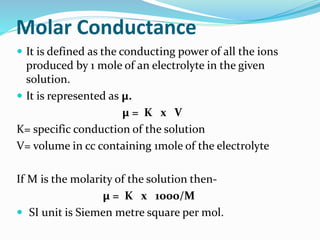
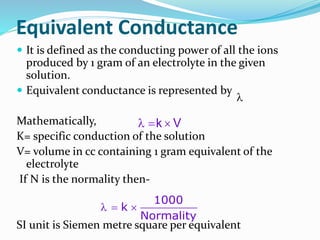
The document discusses the conductance of electrolytic solutions, highlighting the differences between strong and weak electrolytes based on their ionization in water. It defines specific conductance, molar conductance, and equivalent conductance, providing formulas and units for each. Additionally, it explains the effects of dilution and temperature on conductivity as well as Kohlrausch's law regarding limiting molar conductivity.