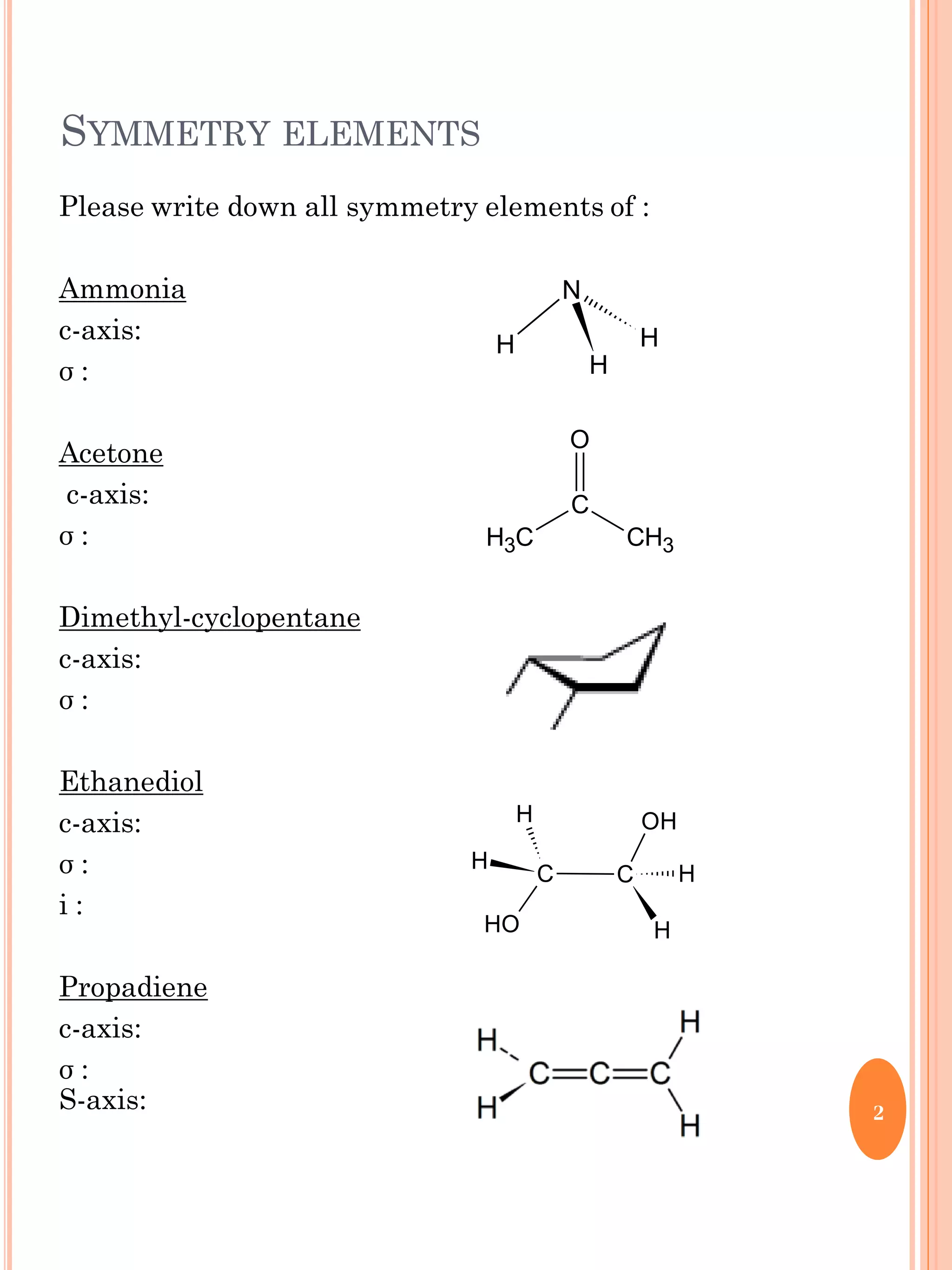
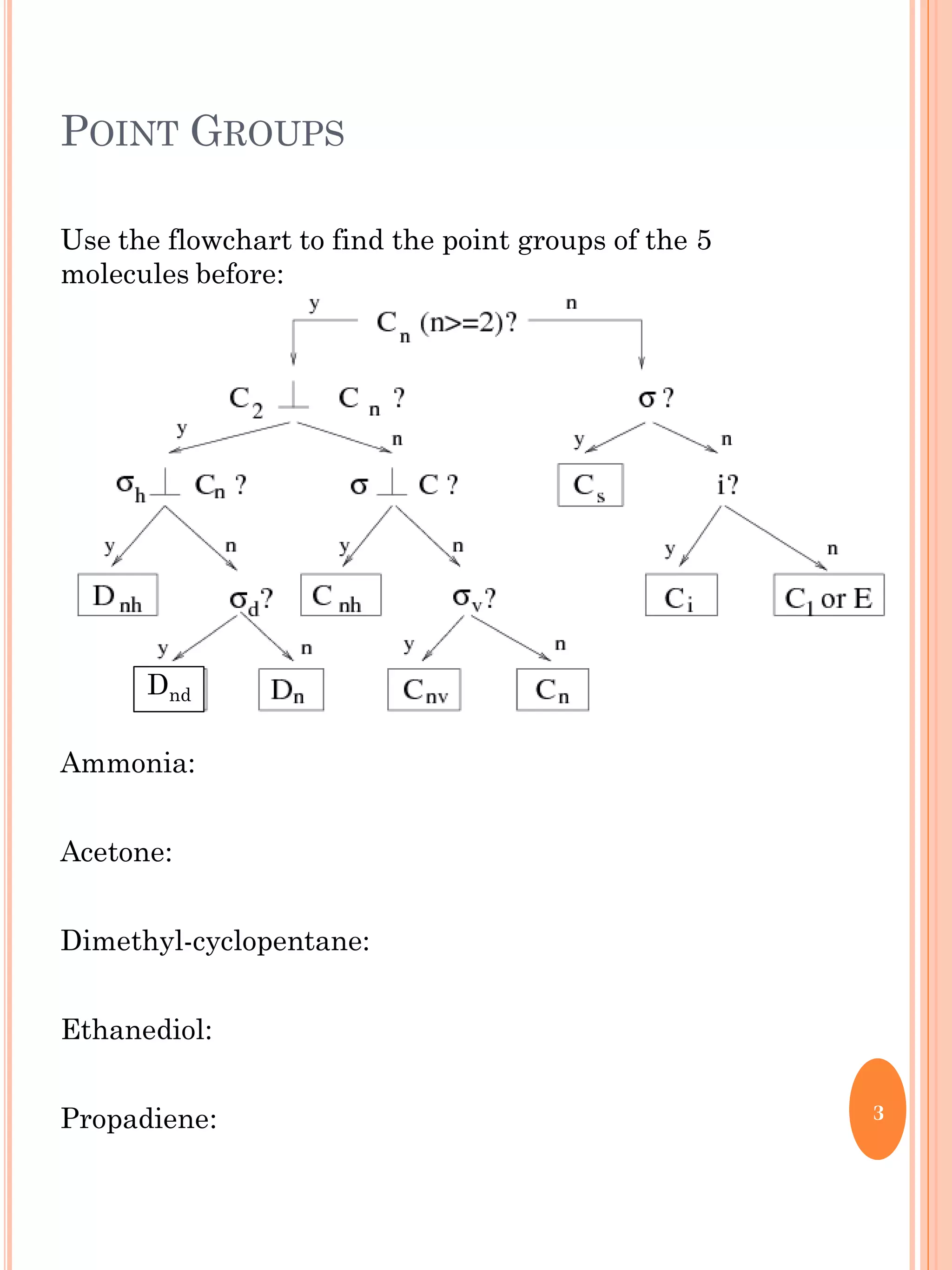
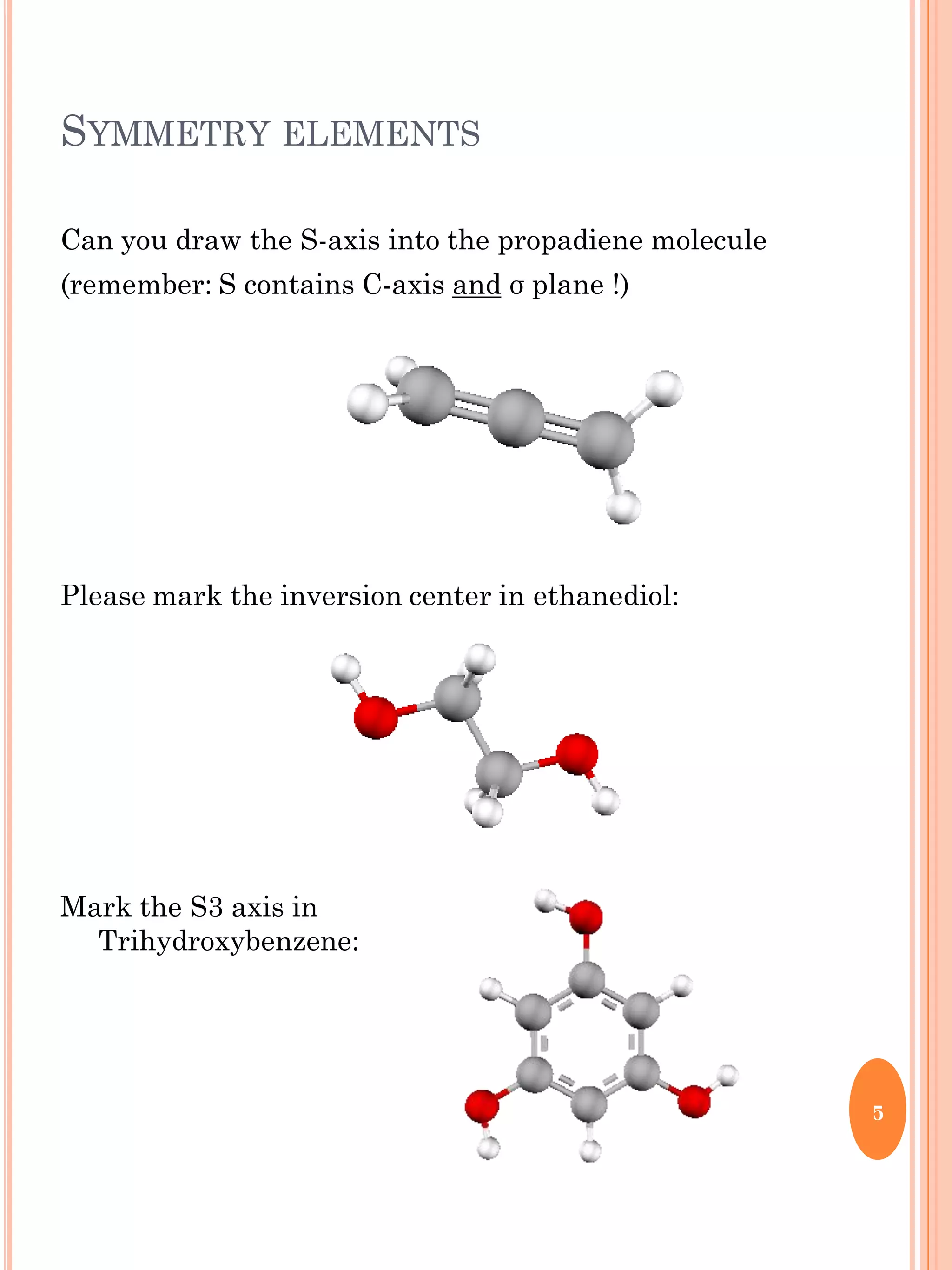
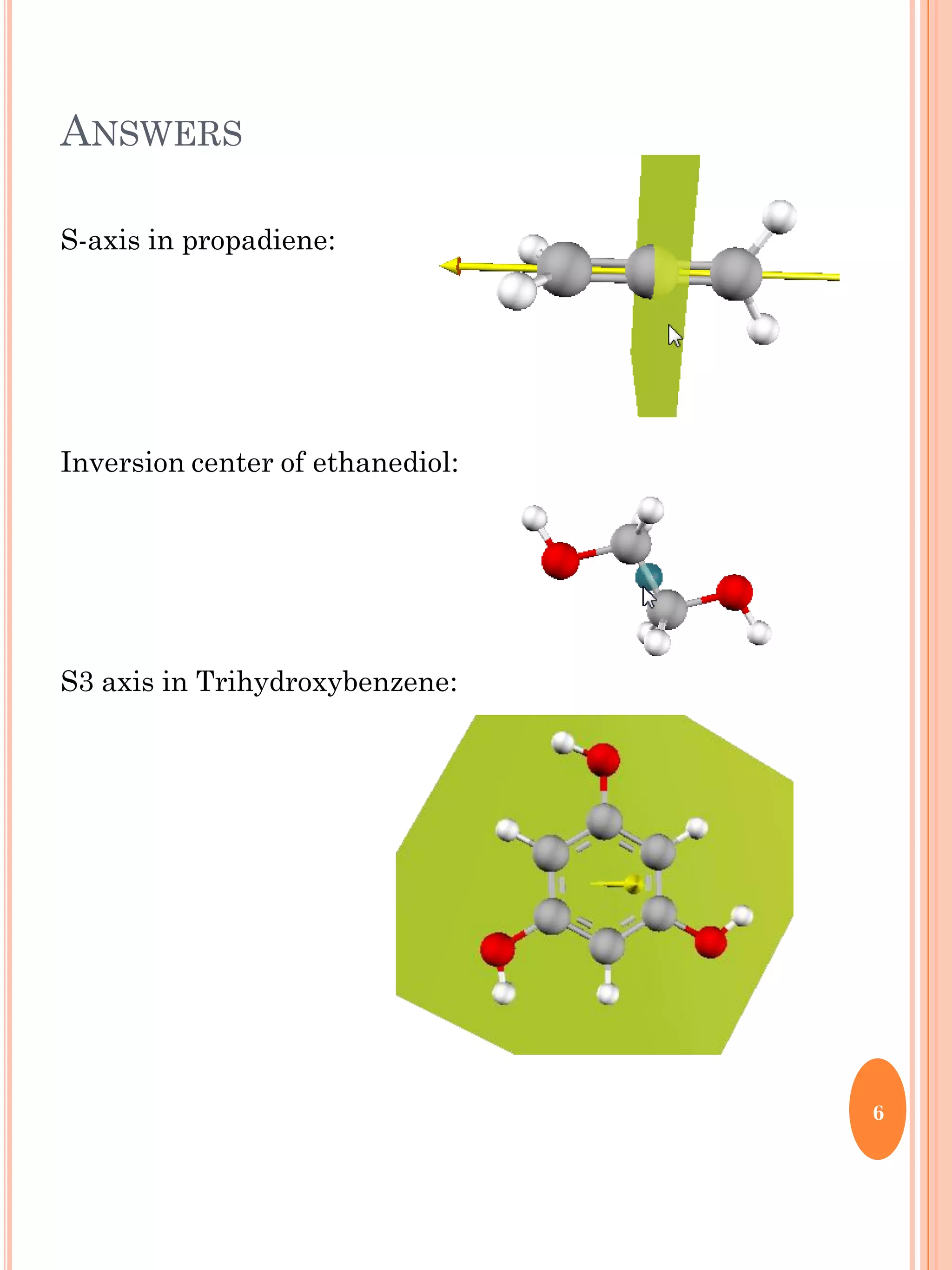
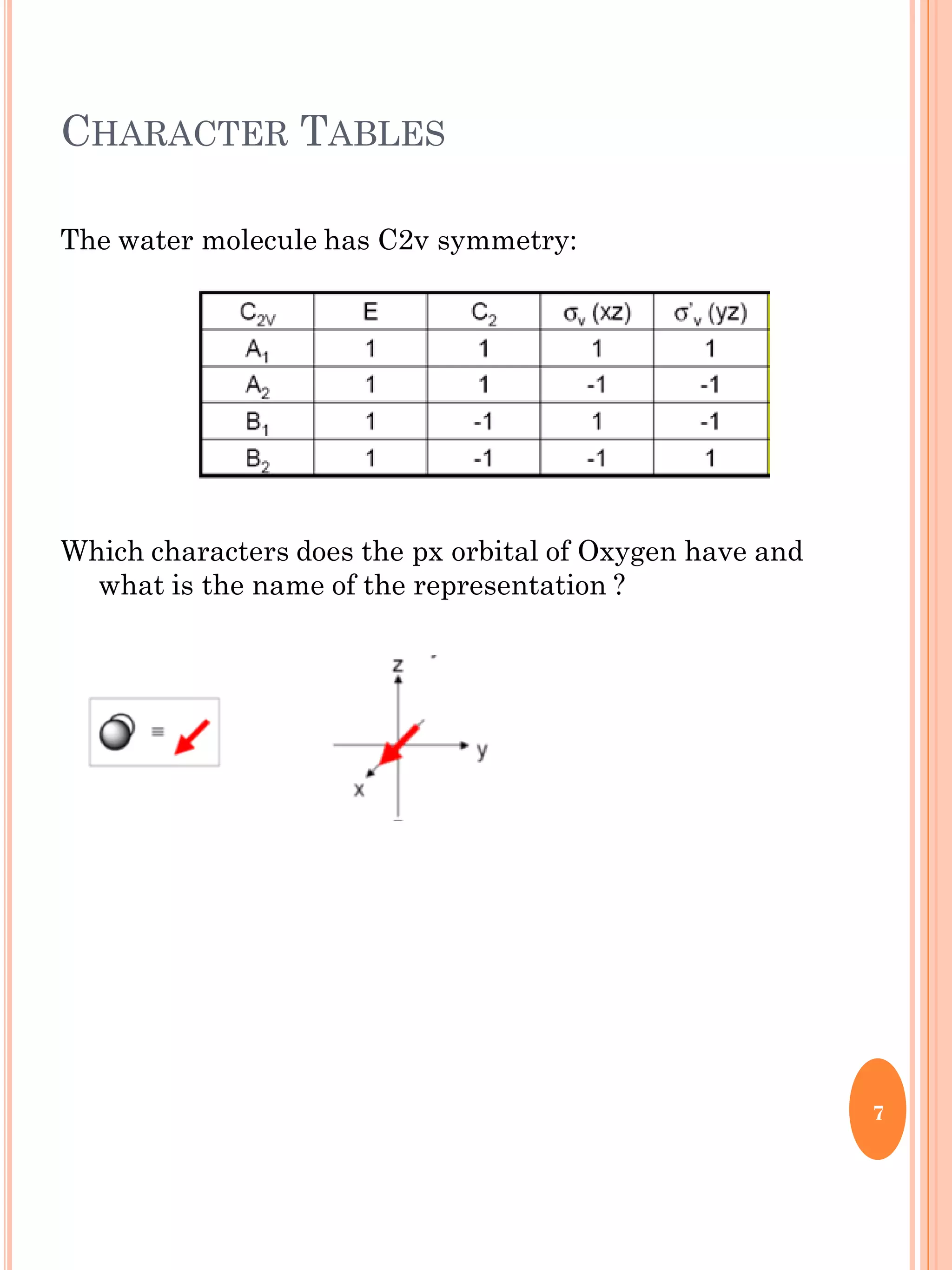
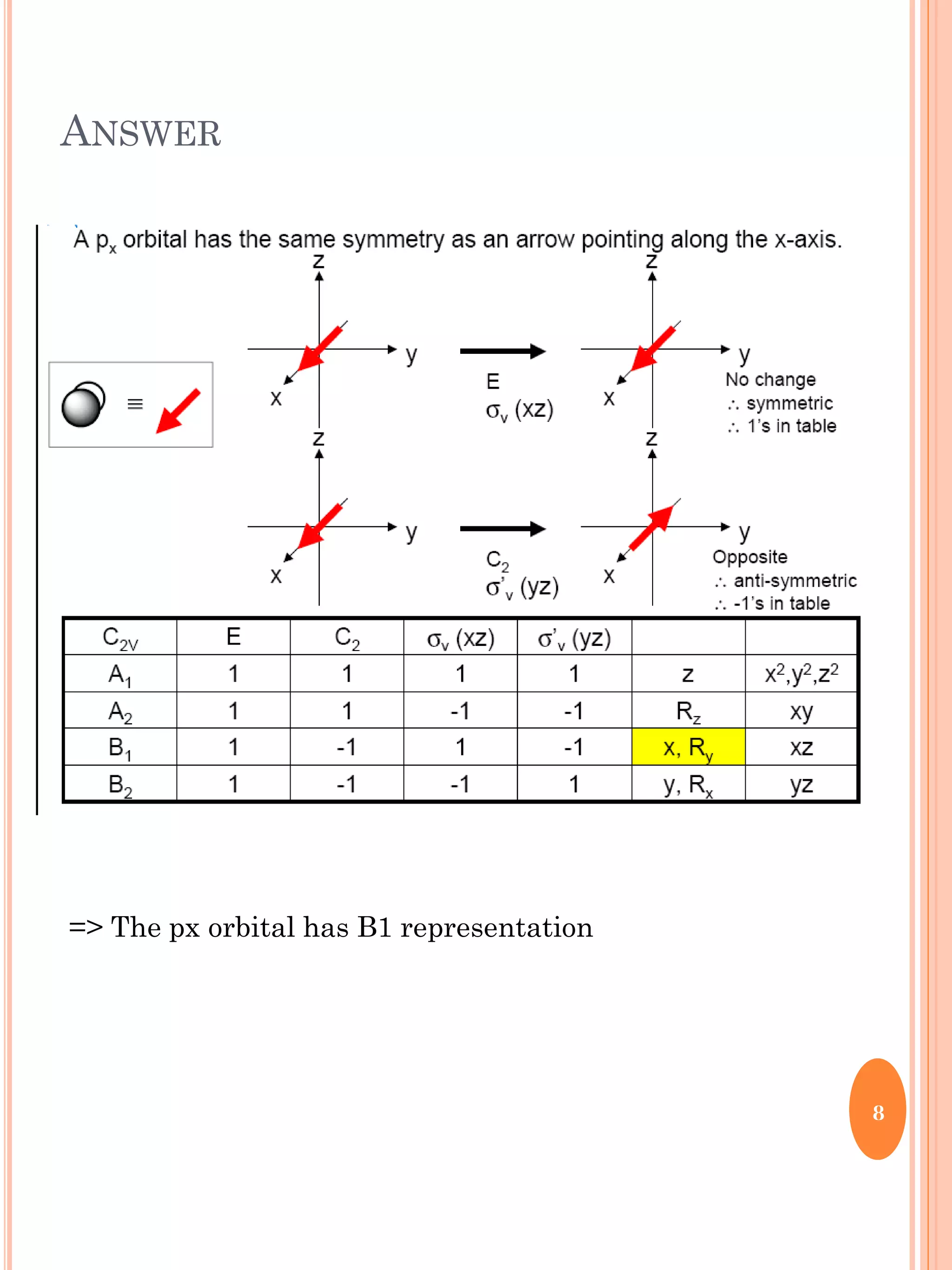
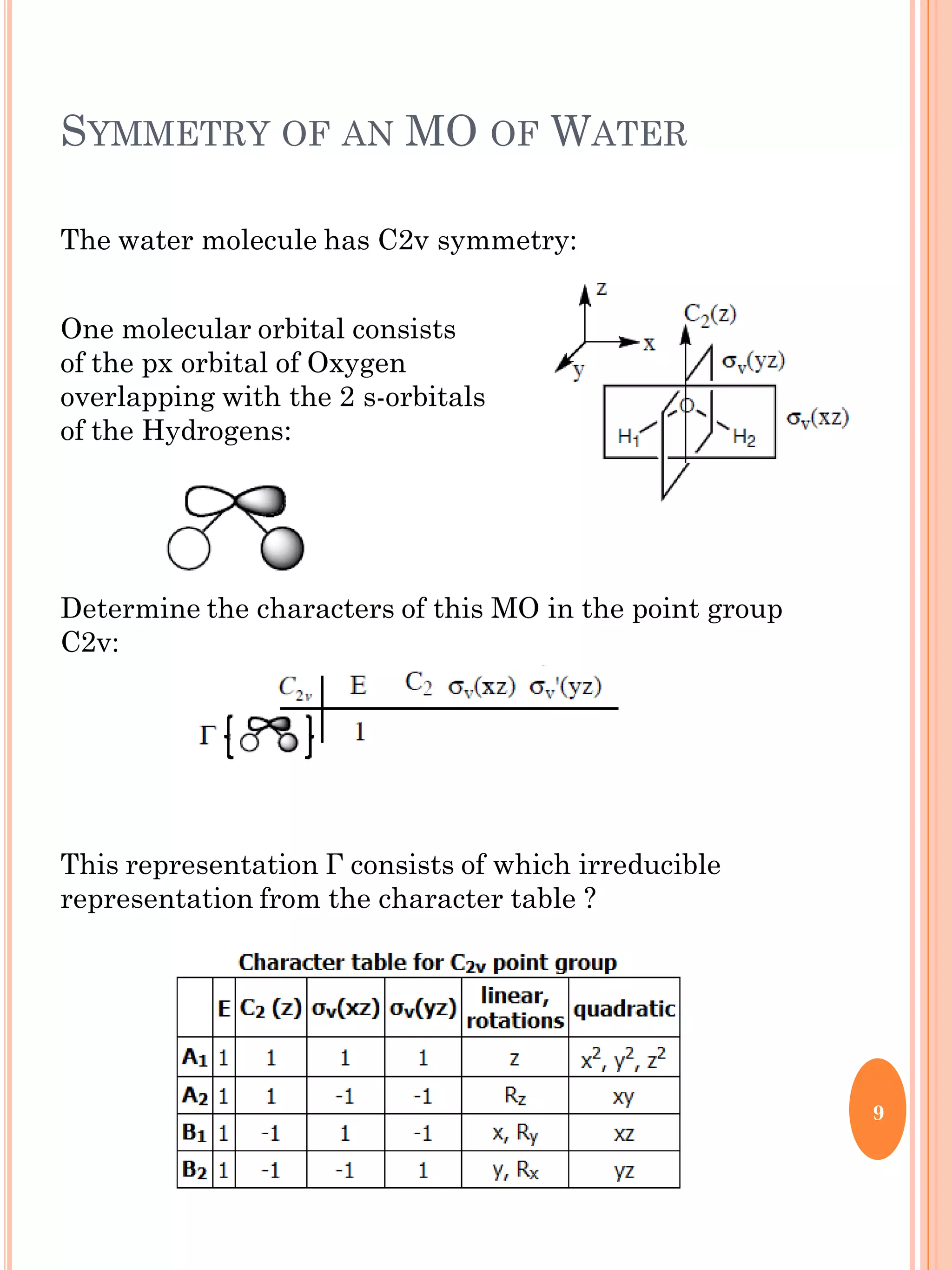
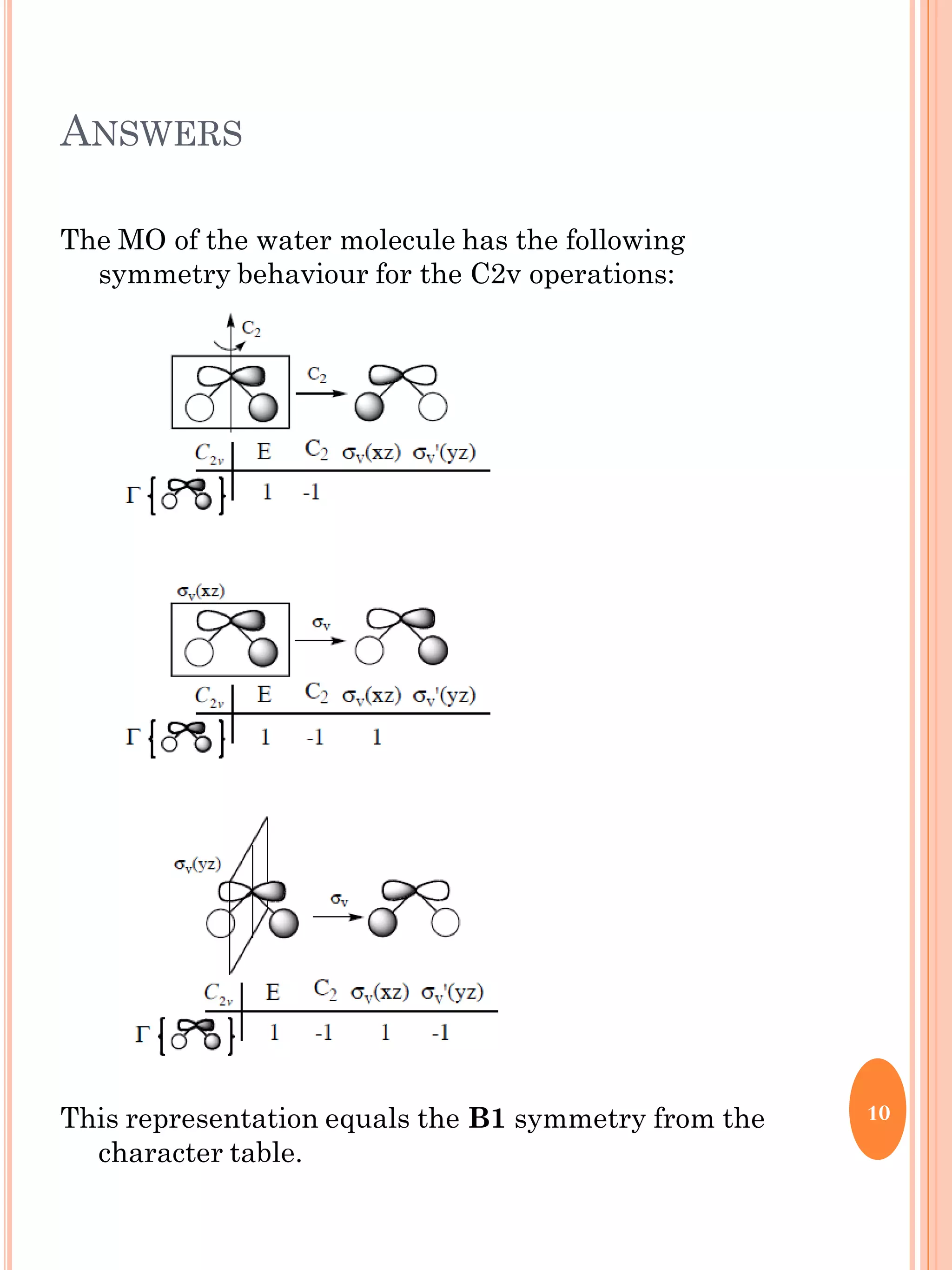
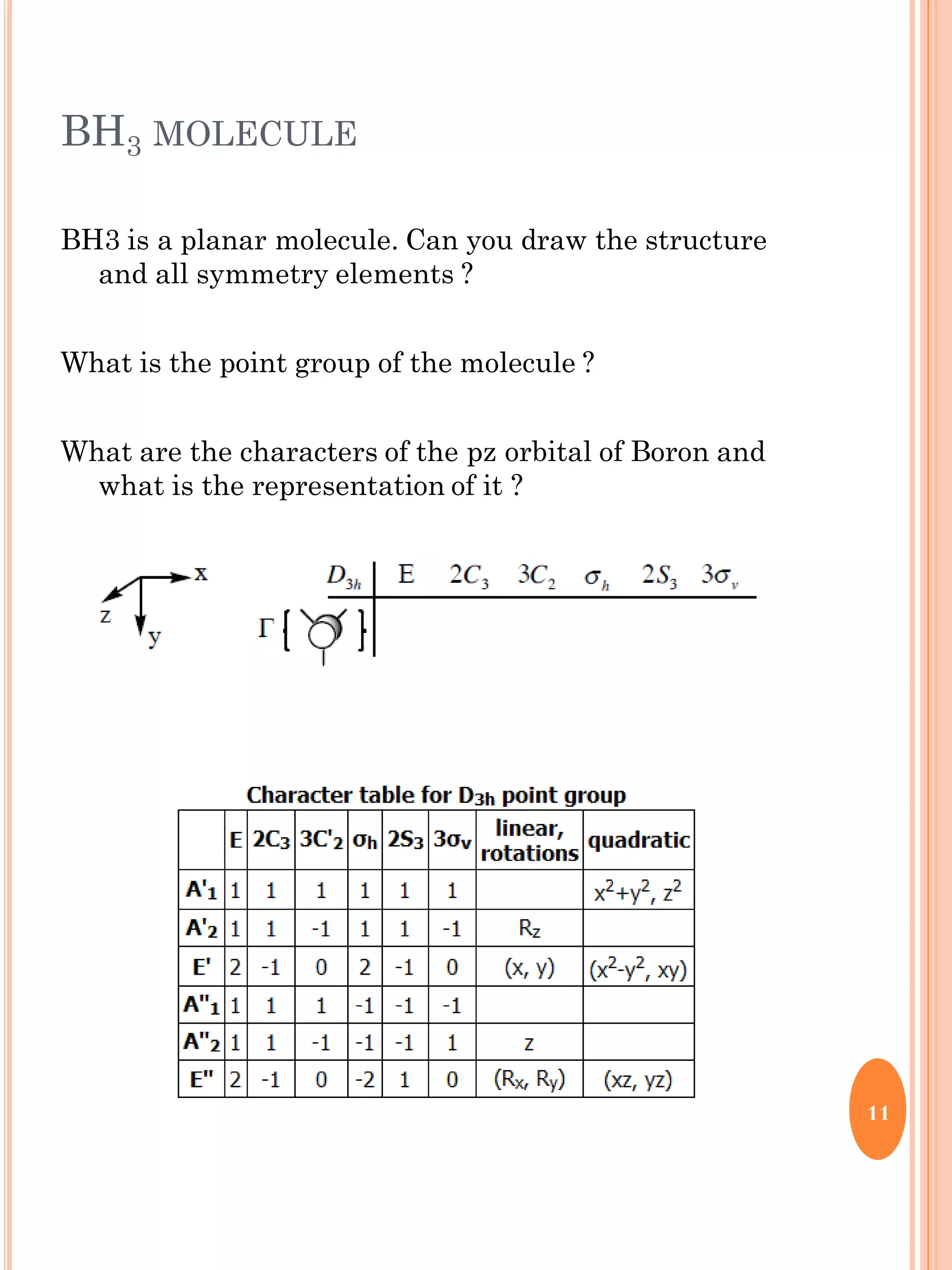
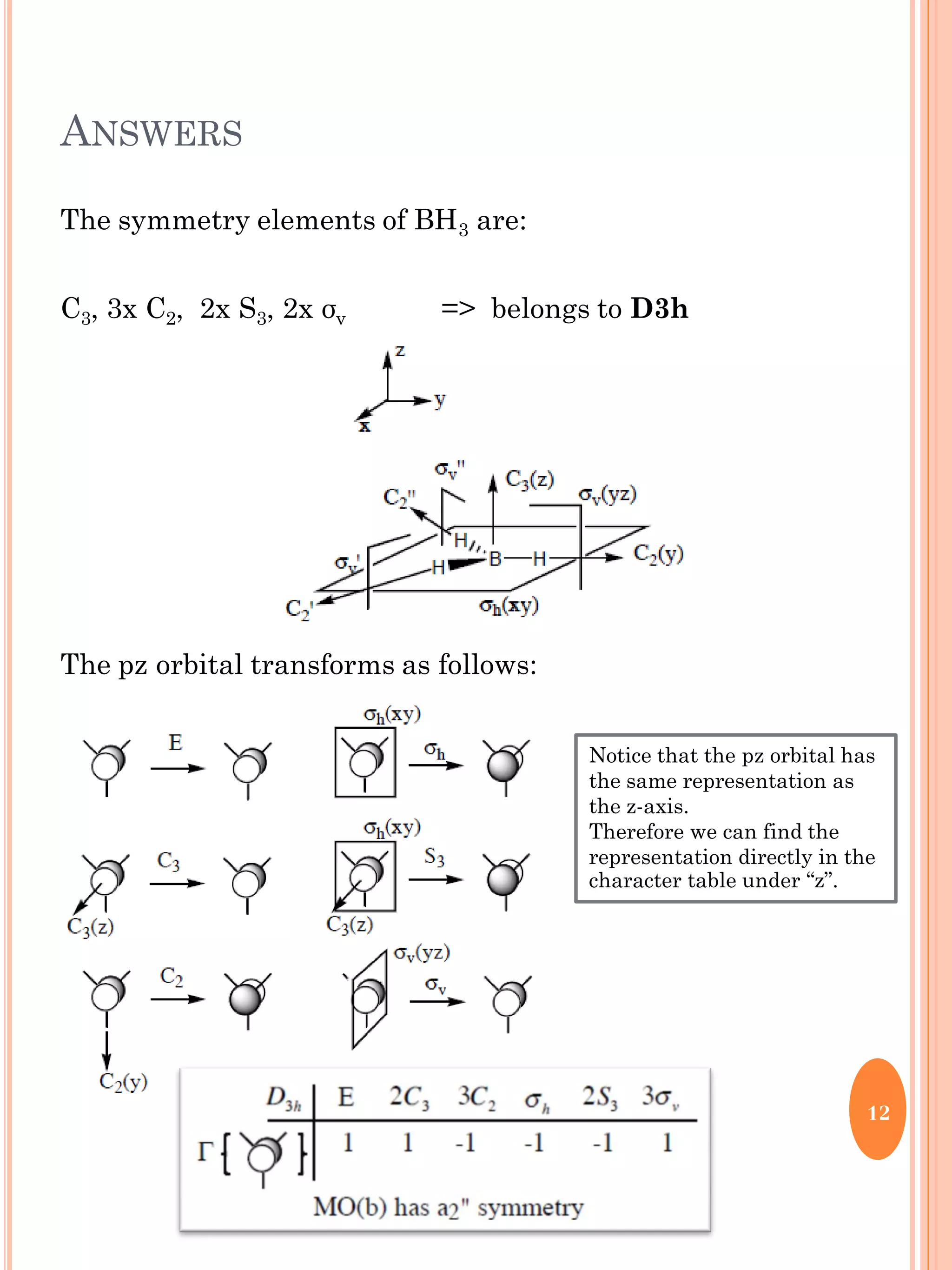
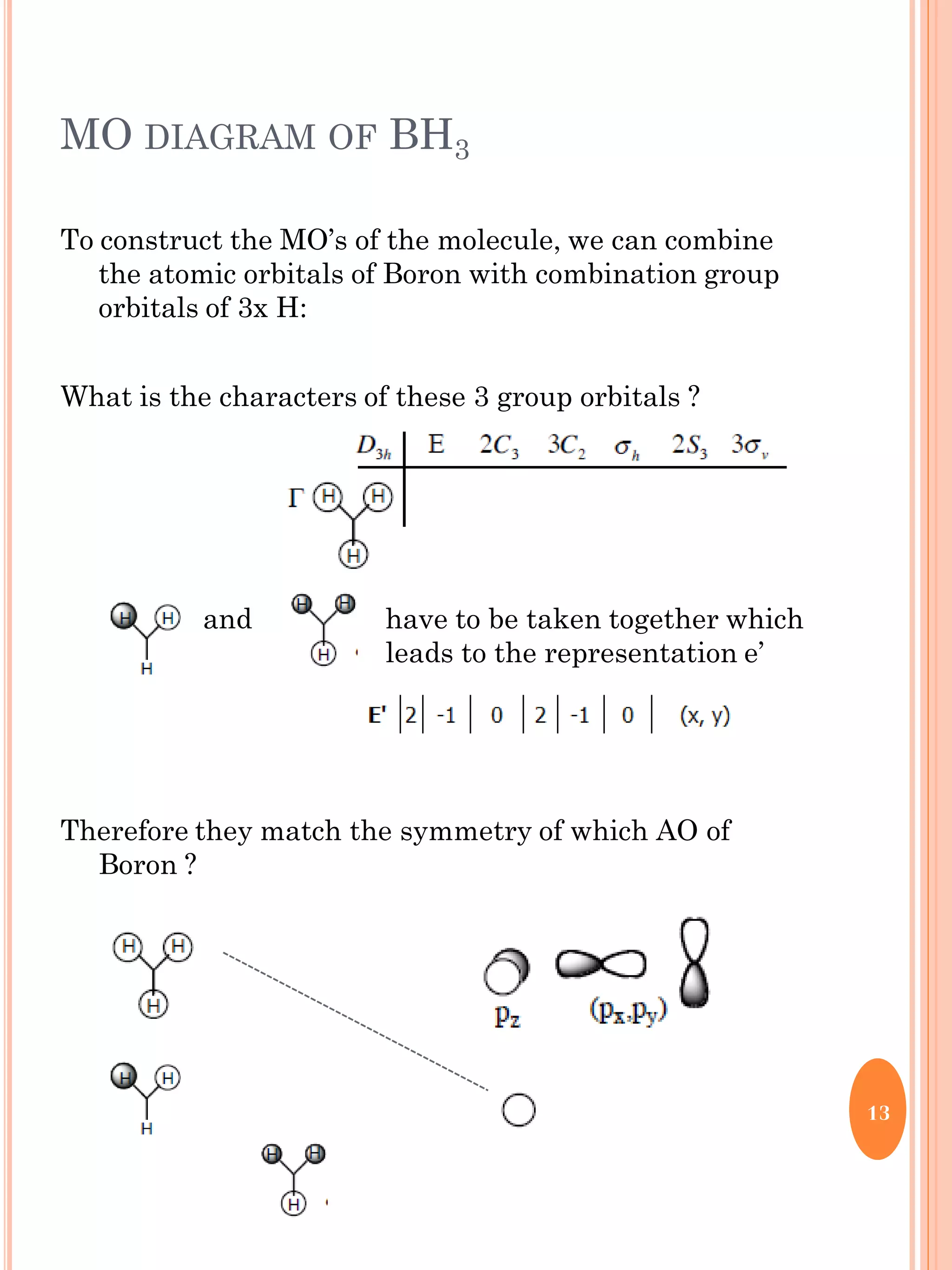
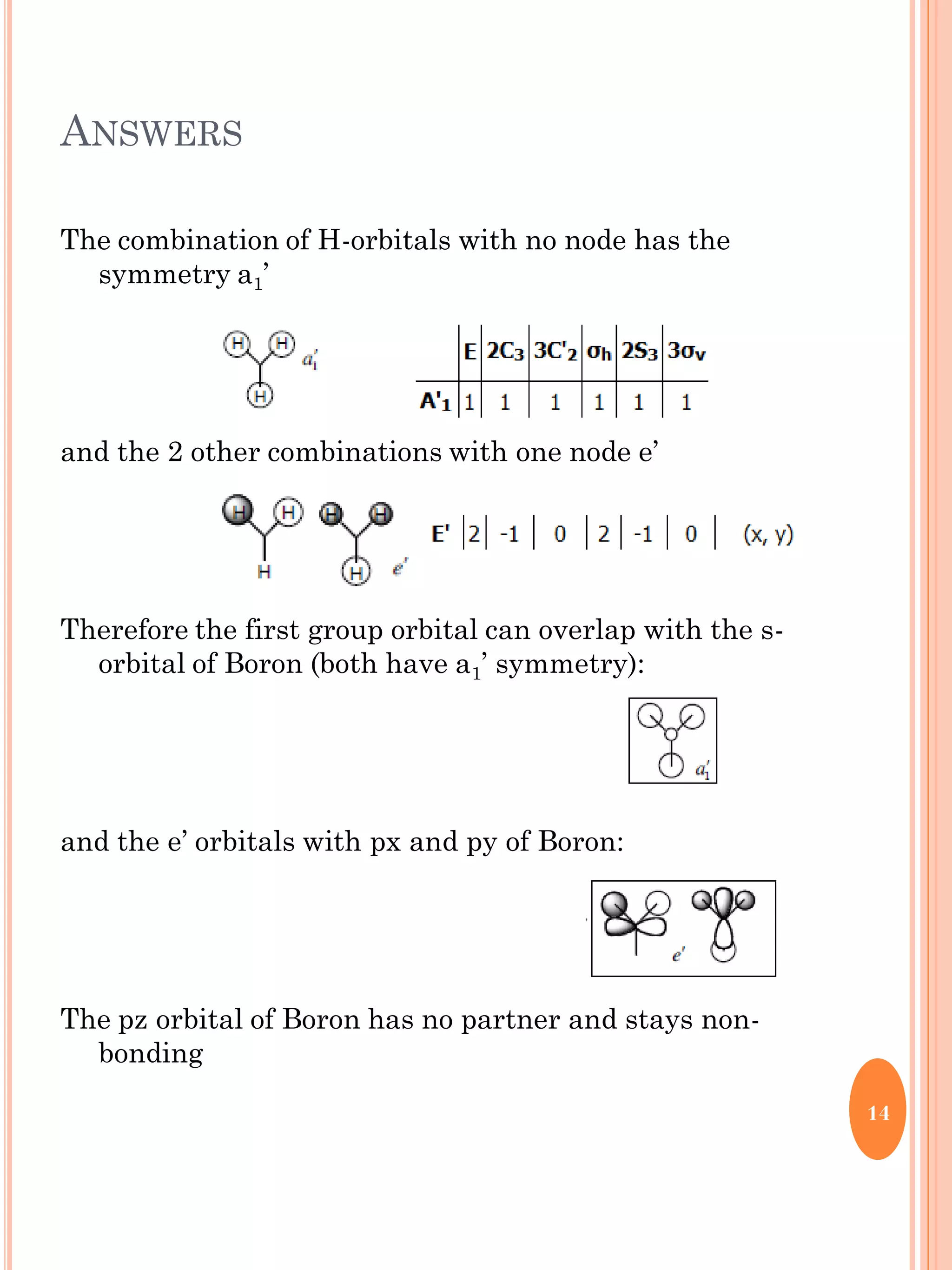
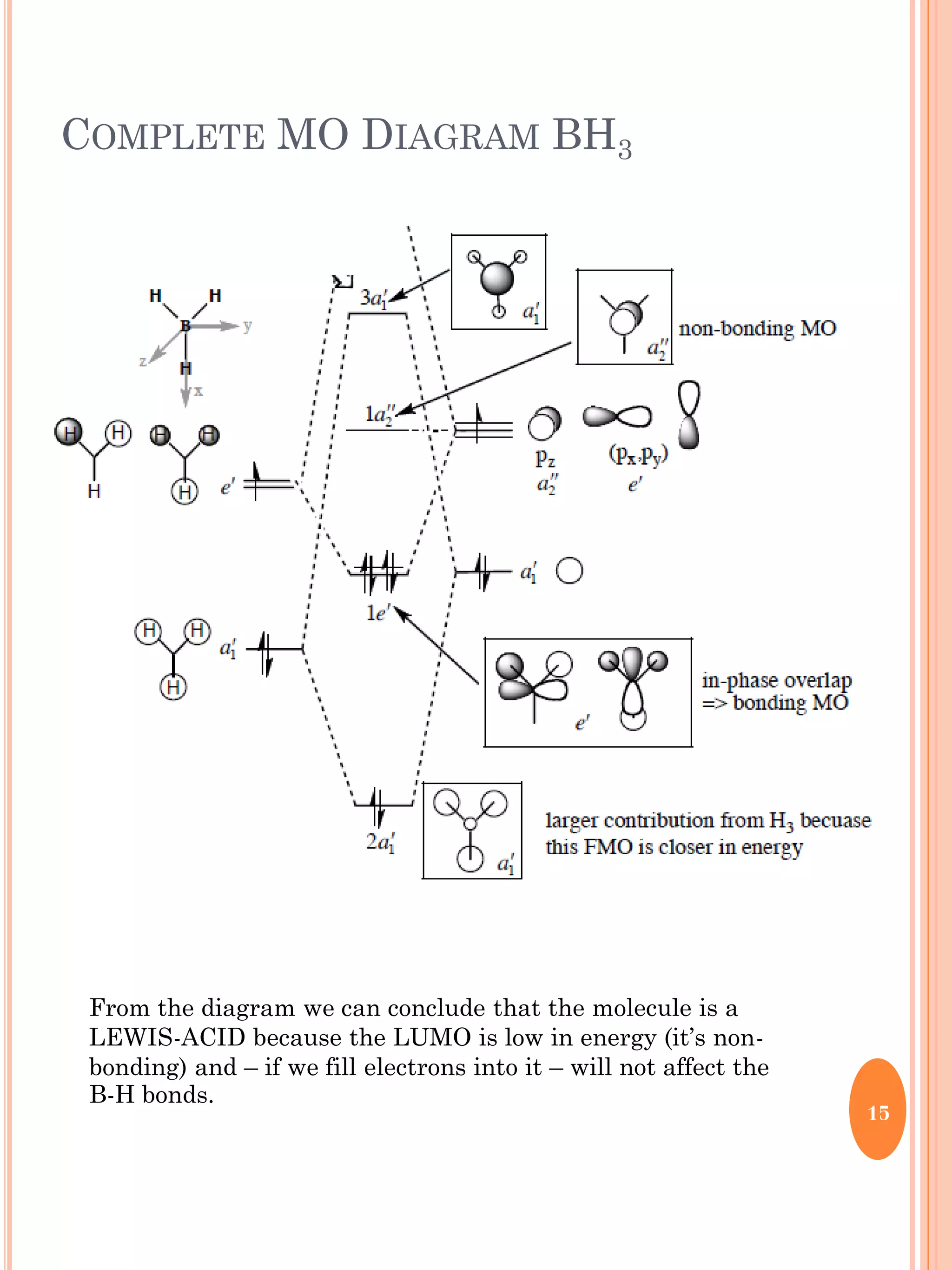
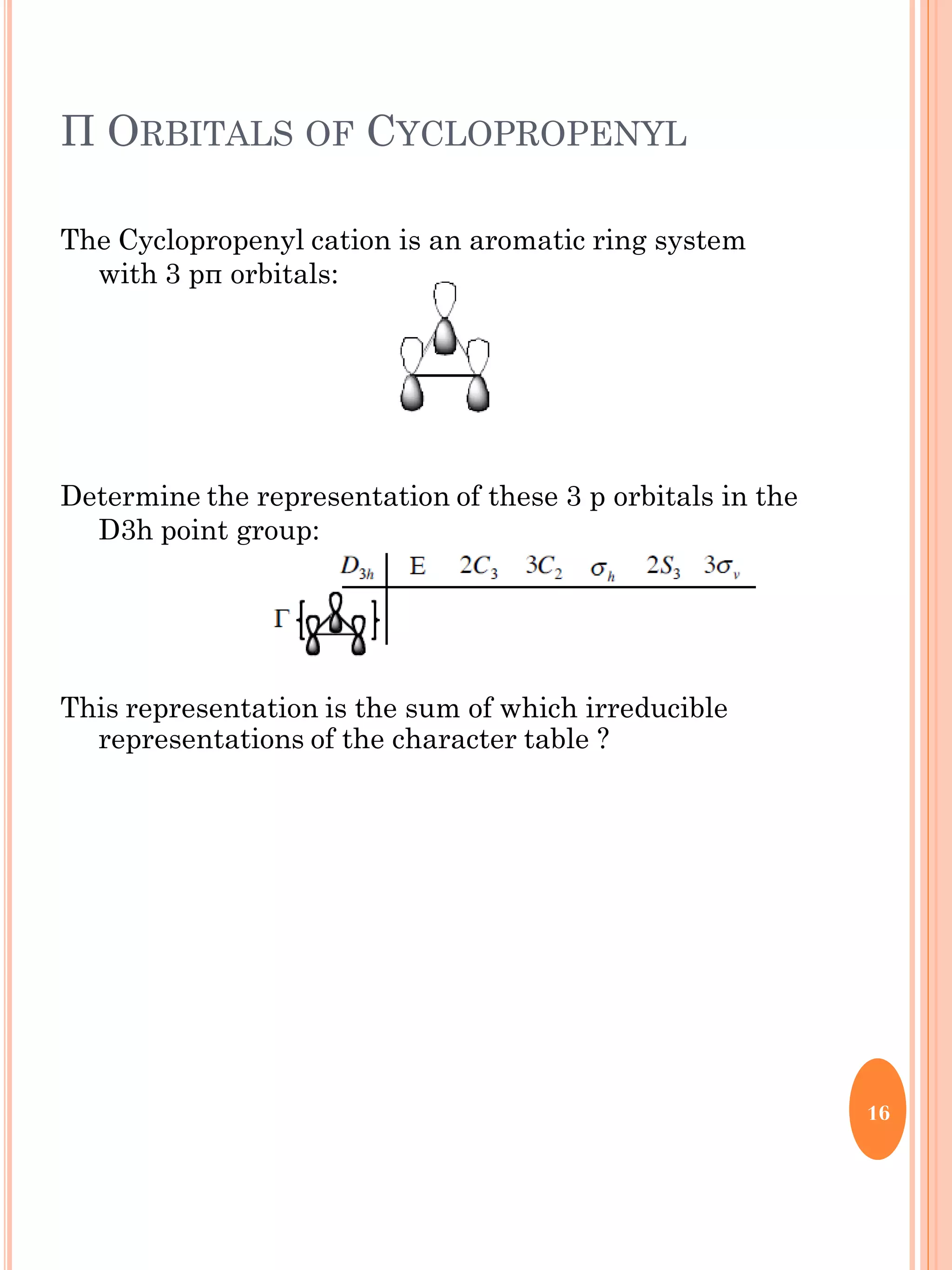
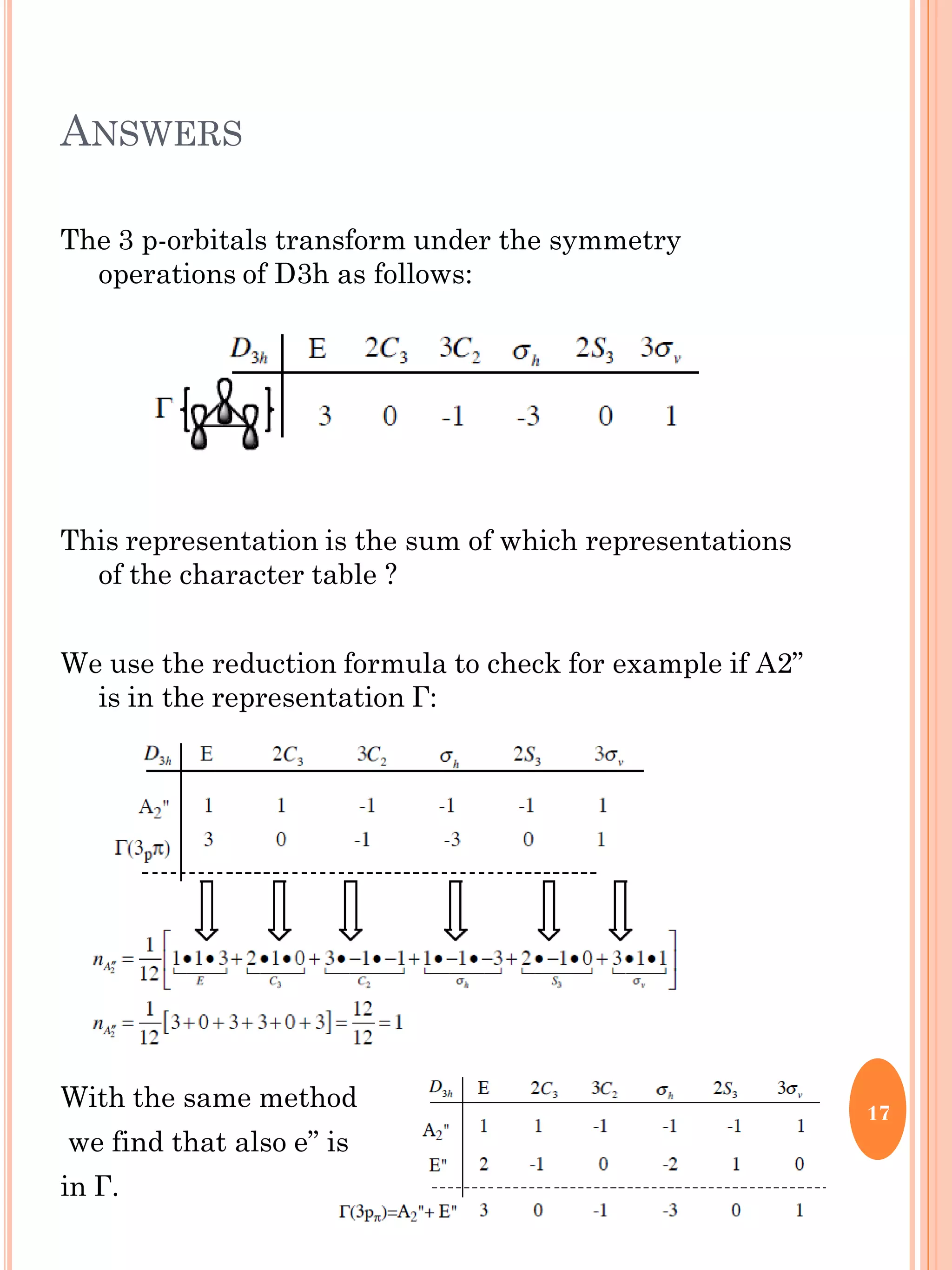
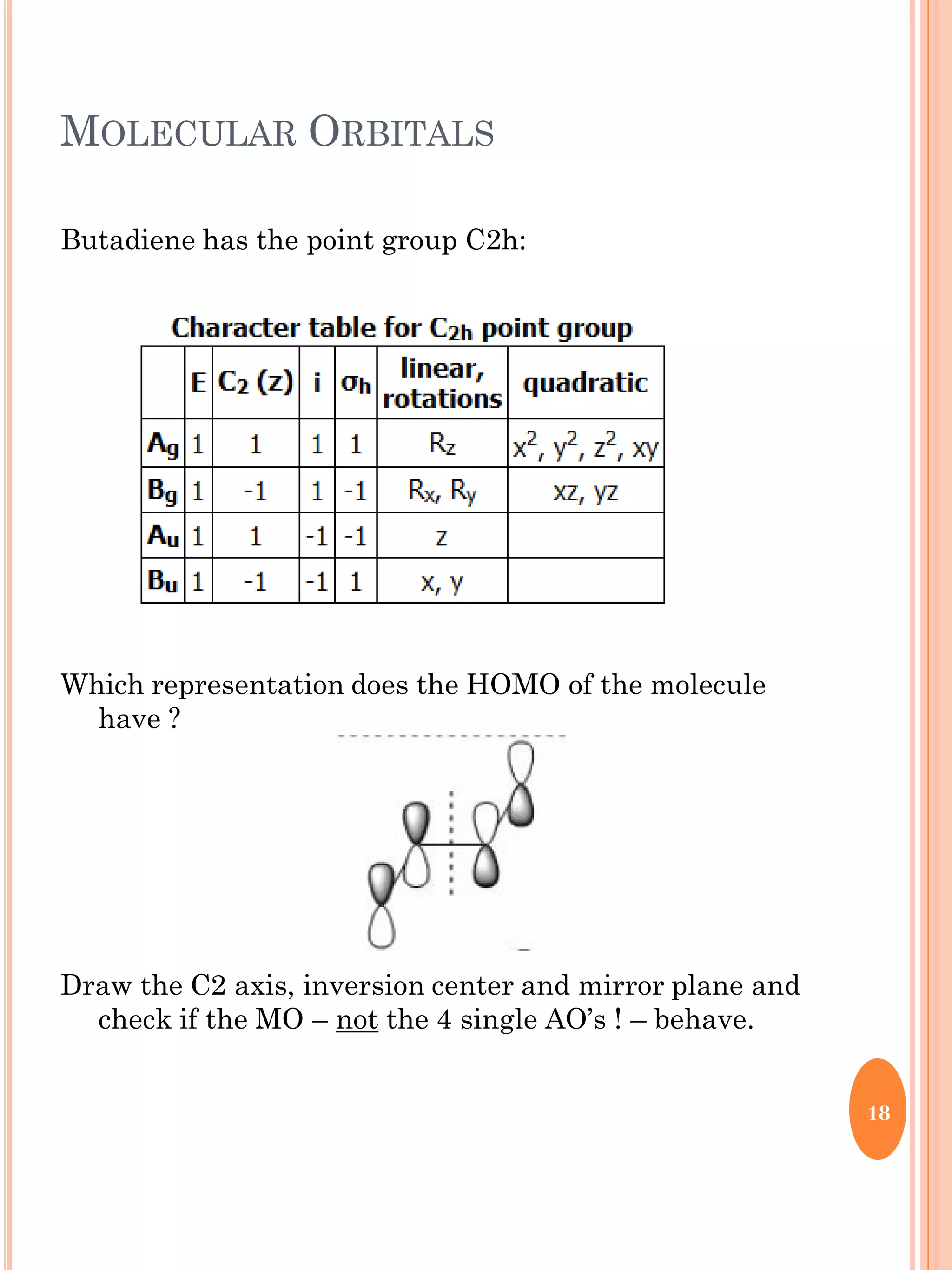
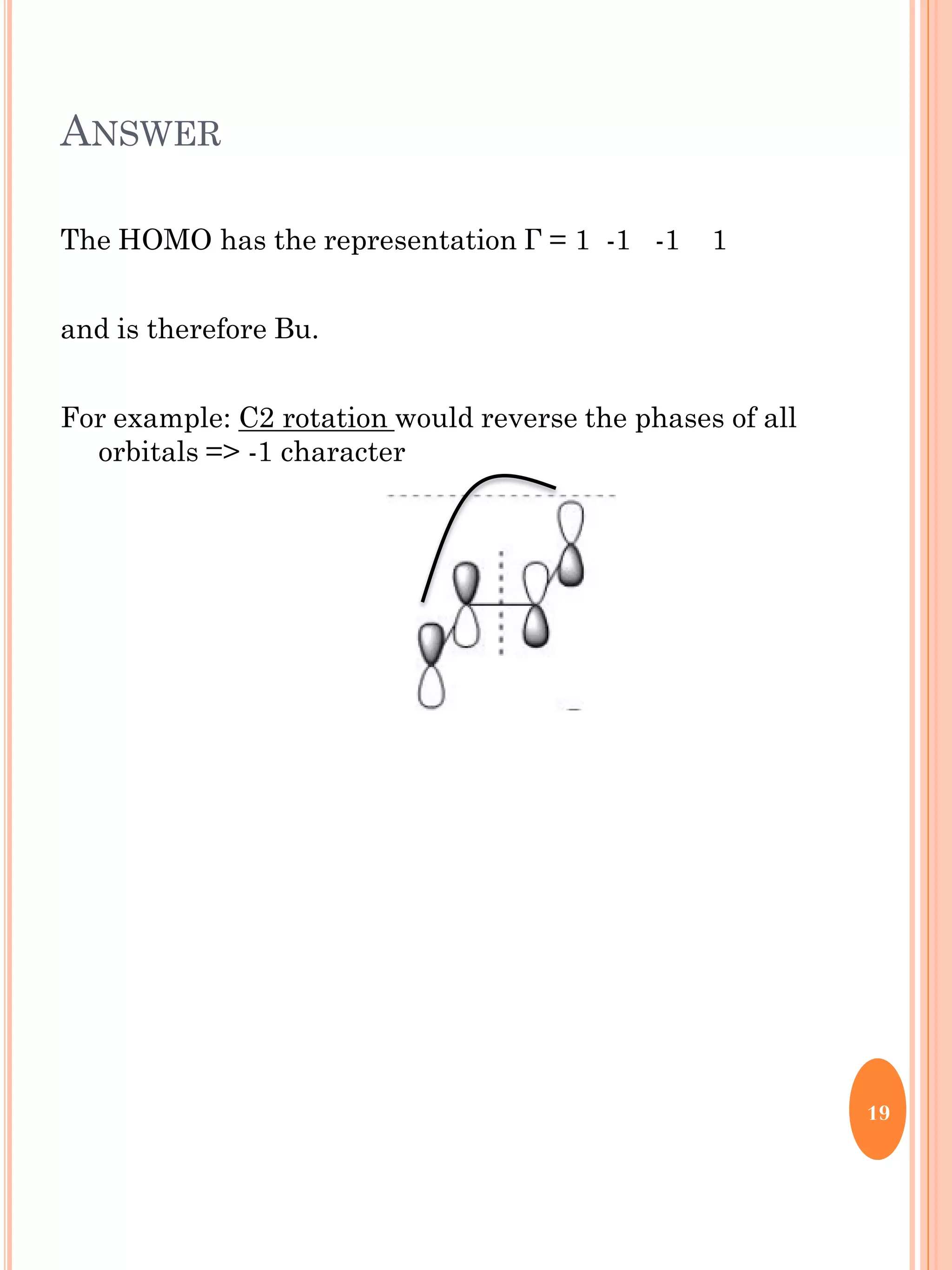
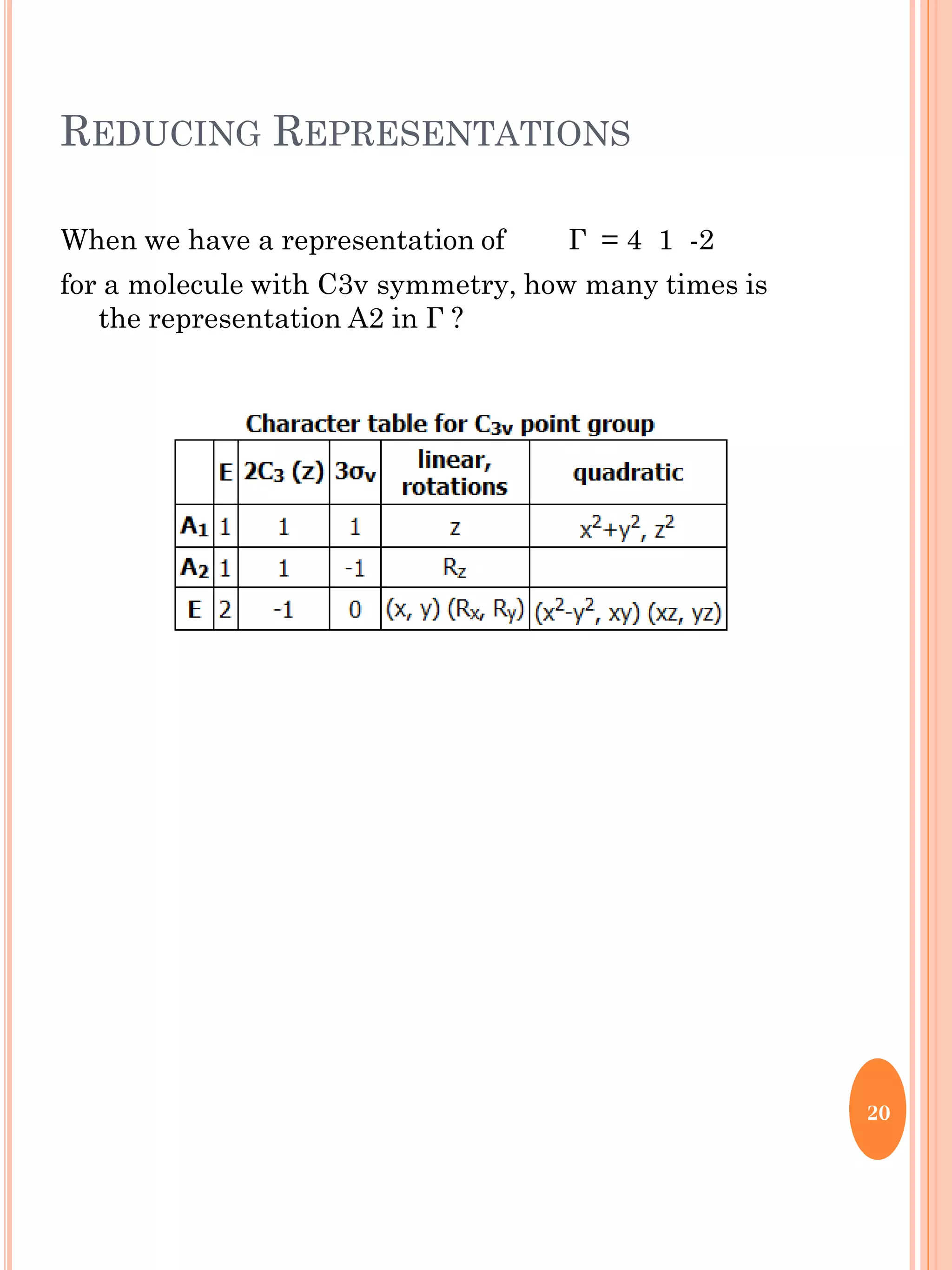
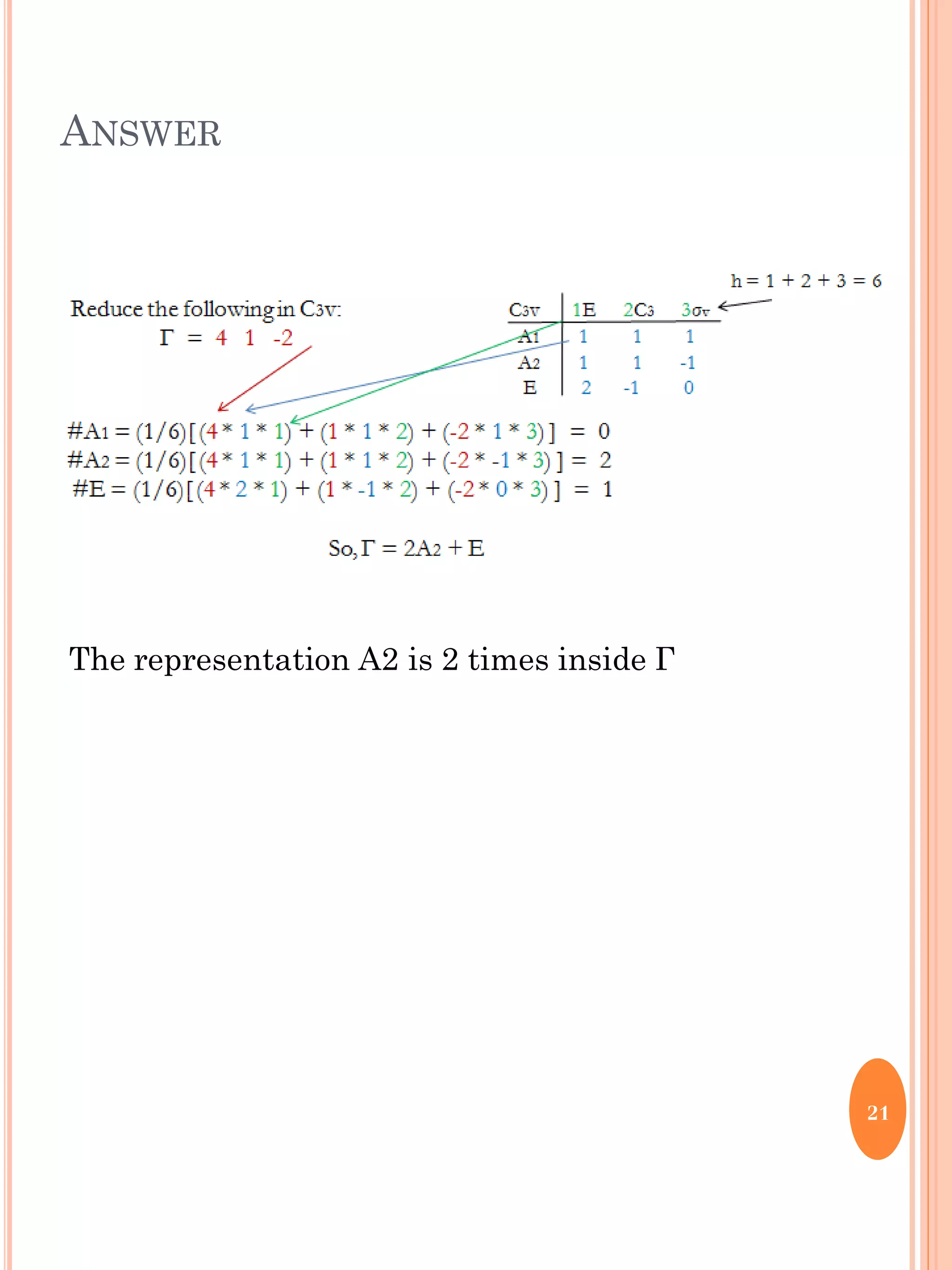
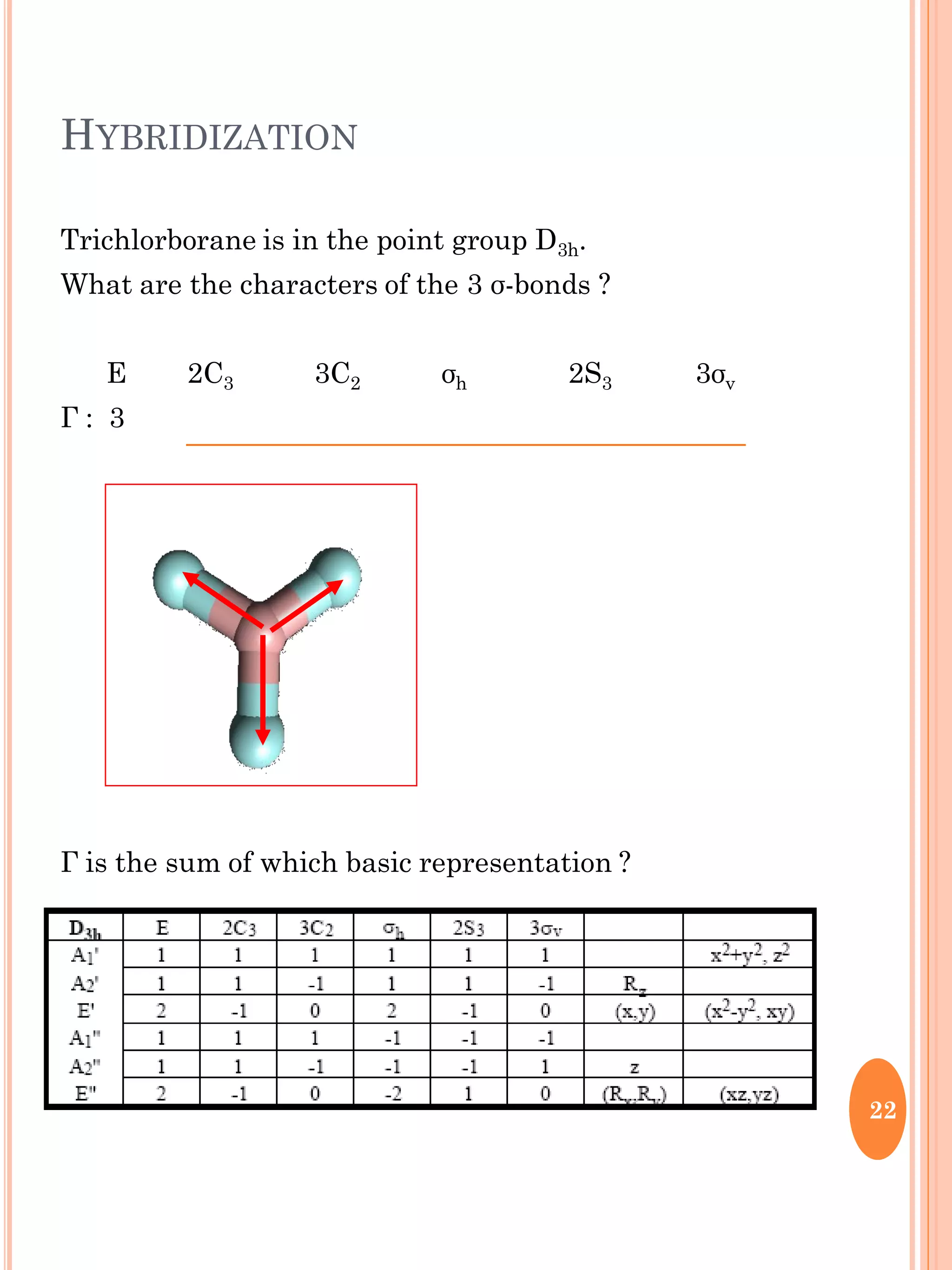
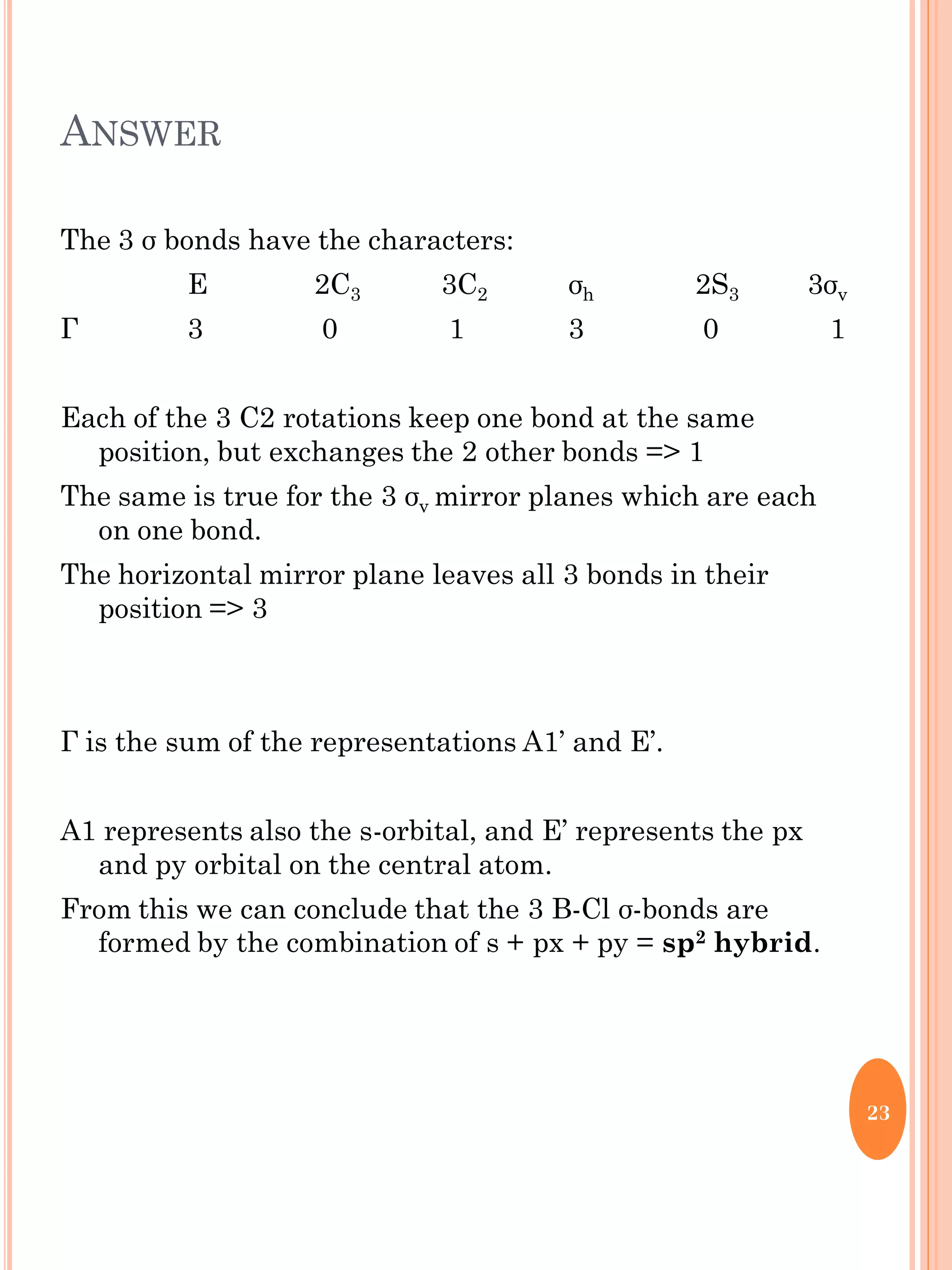
This document discusses group theory and symmetry elements as they relate to several different molecules. It provides examples of identifying the point groups, symmetry elements like rotation axes and planes of inversion, and determining the representations of atomic and molecular orbitals for molecules like ammonia, acetone, ethanediol, propadiene, water, BH3, cyclopropenyl cation, butadiene, and trichlorborane. Worked examples are provided to demonstrate how to analyze symmetry properties and construct molecular orbital diagrams for various systems.