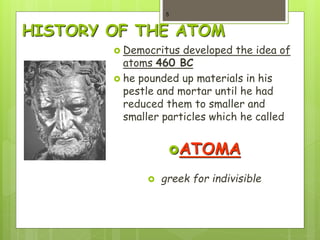
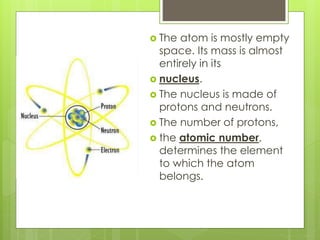
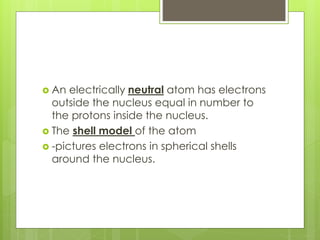
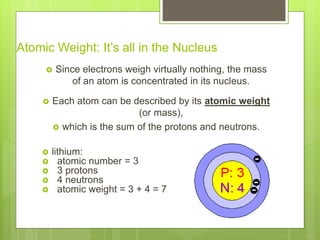
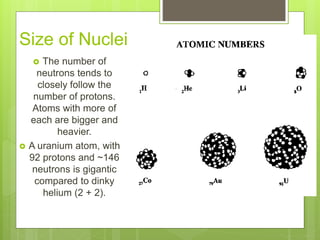
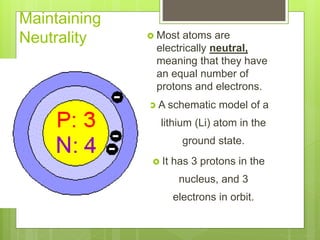
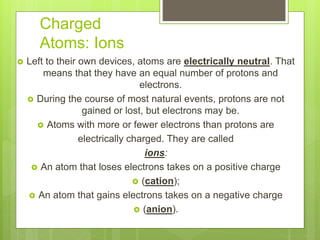
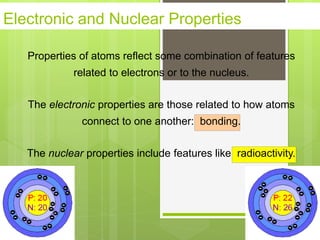
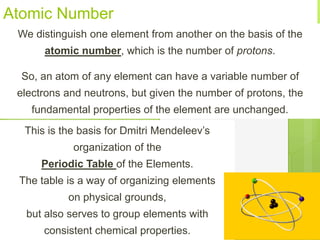
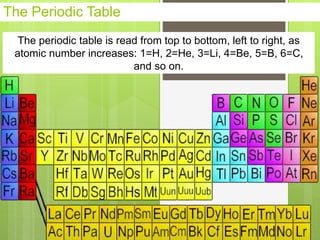
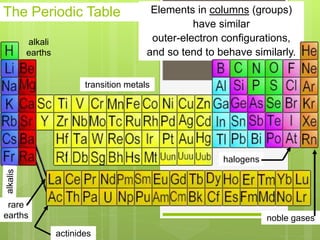
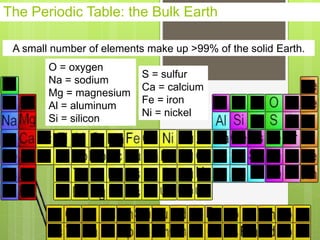
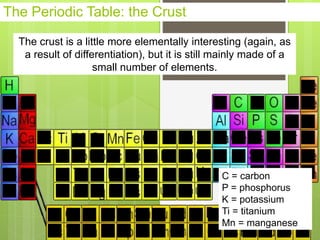
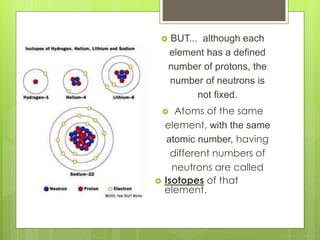
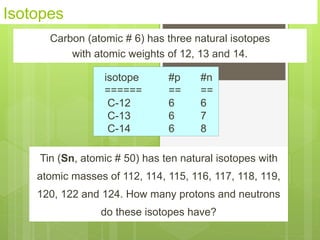
1. Atoms are the building blocks of matter and are made up of a nucleus containing protons and neutrons surrounded by electrons.
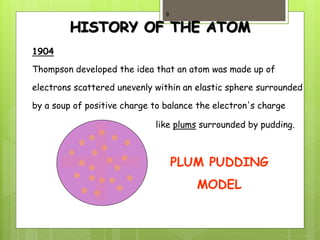
2. Early atomic models included John Dalton's billiard ball model in 1800 and J.J. Thompson's plum pudding model in 1904 which depicted electrons suspended in a positively charged sphere.
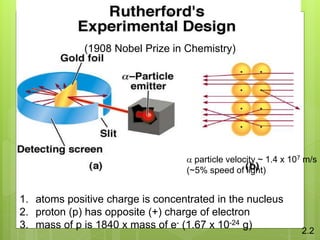
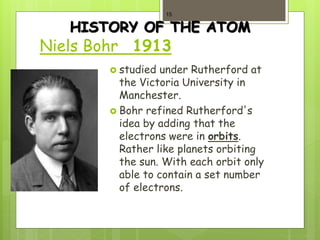
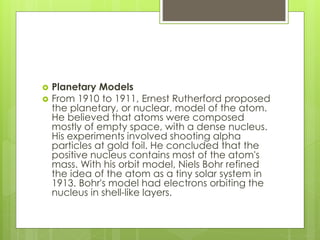
3. In 1911, Ernest Rutherford proposed the nuclear model from his gold foil experiment results, and Niels Bohr refined it in 1913 to show electrons in distinct orbitals around the nucleus similar to planets revolving around the sun.