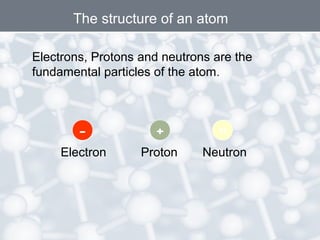
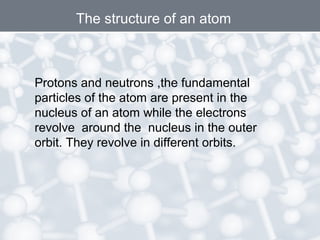
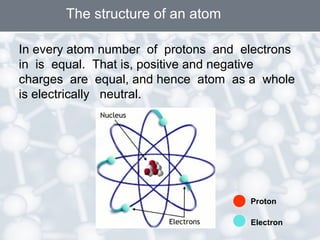
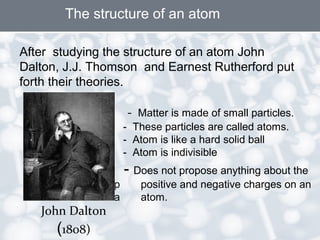
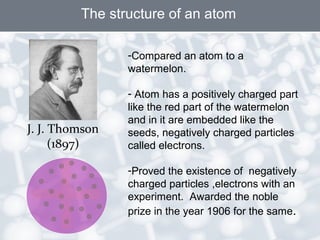
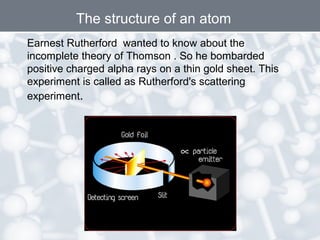
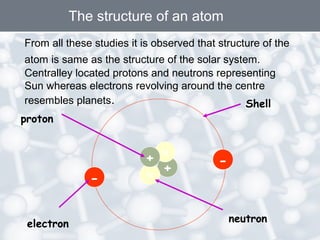
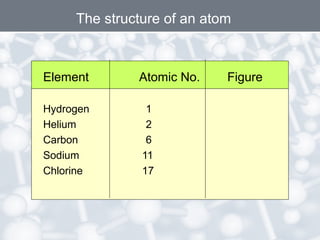
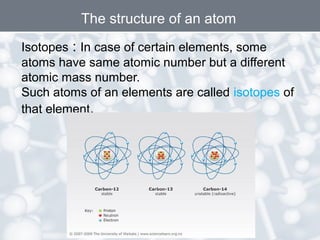
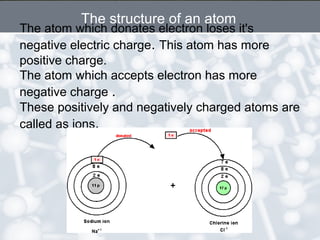
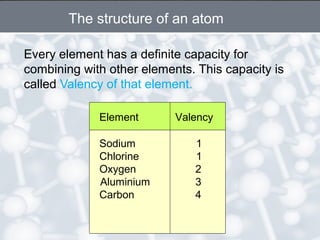
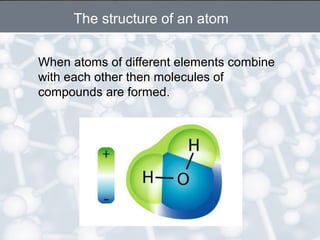
The document discusses the structure of an atom. It explains that an atom contains protons, neutrons, and electrons. Protons and neutrons are located in the nucleus, while electrons orbit the nucleus. John Dalton, J.J. Thomson, and Ernest Rutherford contributed theories to our understanding of atomic structure through experiments. Rutherford determined that the nucleus is very small and contains a positive charge, with electrons orbiting the outside. The number of protons defines the atomic number of an element.