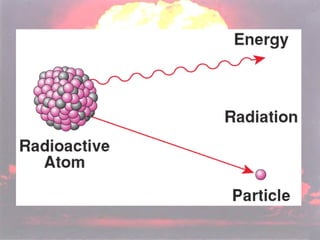
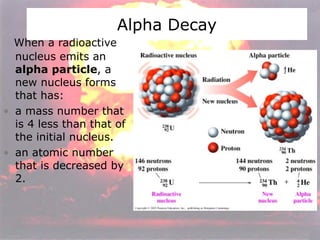
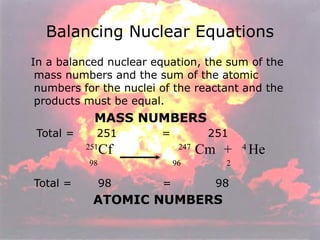
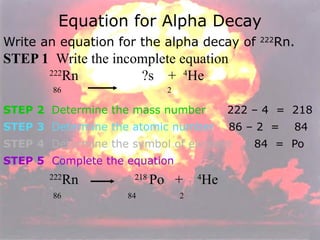
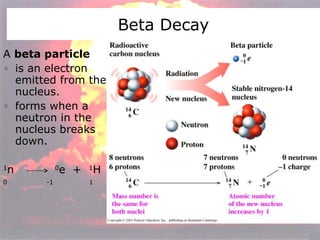
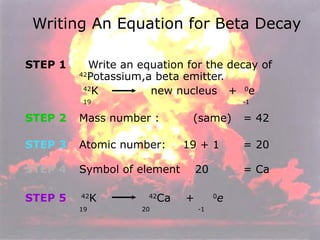
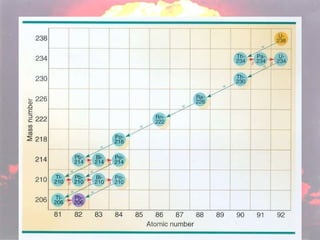
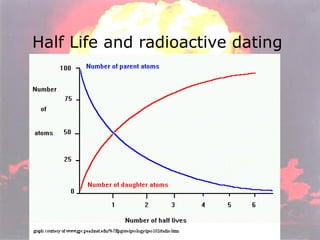
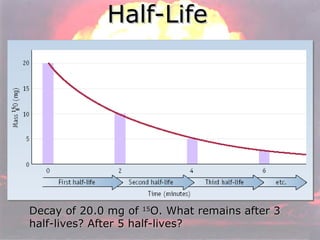
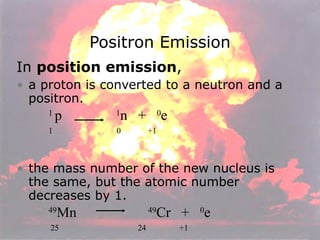
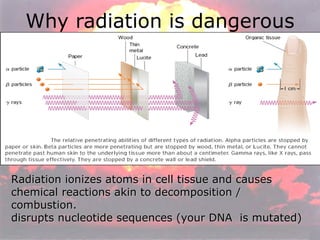
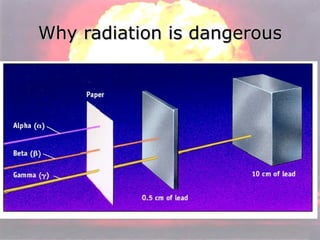
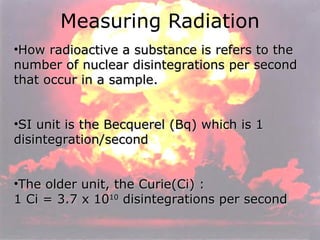
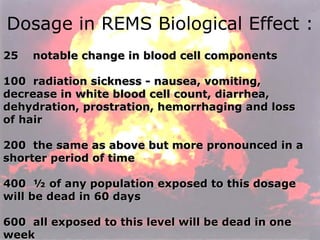
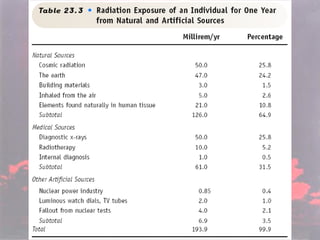
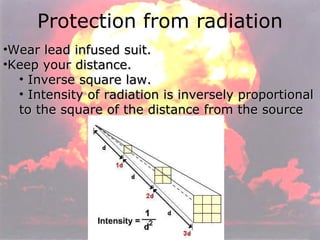
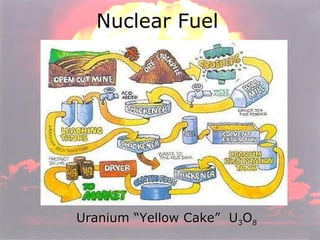
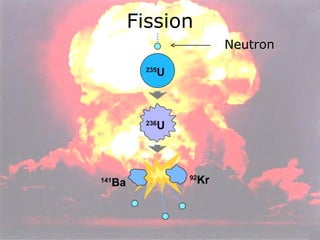
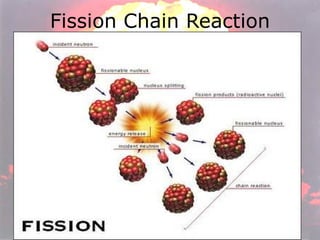
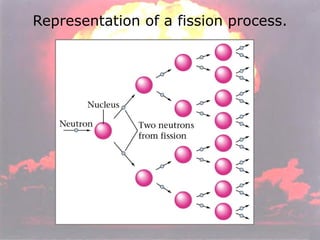
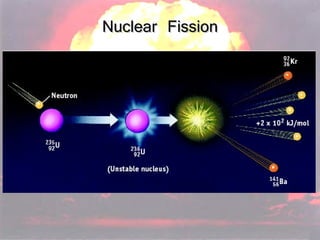
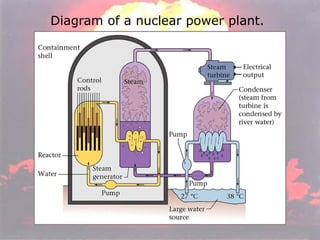
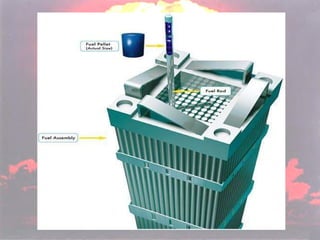
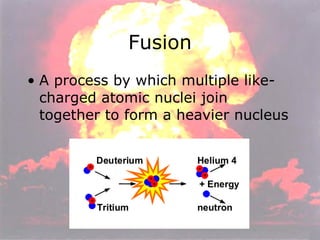
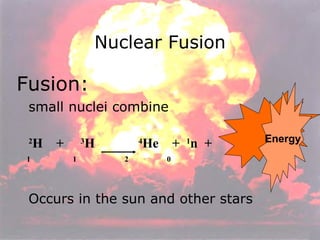
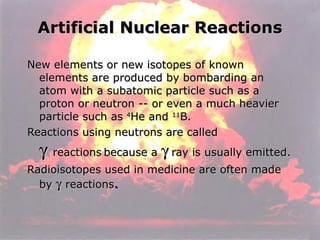
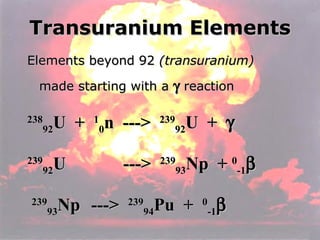
Marie Curie discovered radioactivity through her work on atoms and their structure. Nuclear reactions involve changes to the nucleus through loss of particles and rearrangement of protons and neutrons, releasing significant energy. There are three main types of radiation emitted in radioactive decay: alpha, beta, and gamma. Half-life refers to the time it takes for half of a radioactive sample to decay and is used in radioactive dating. Radiation is dangerous as it can ionize atoms and damage DNA, disrupting cells.