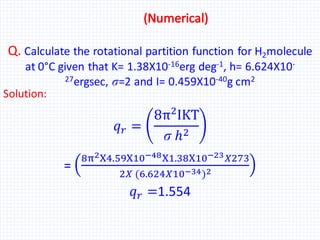
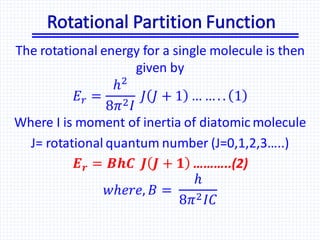
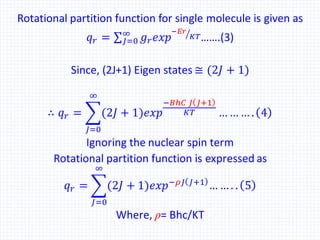
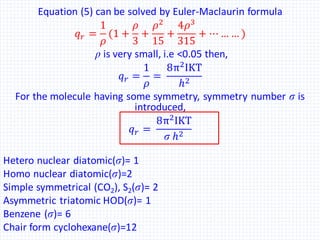
The document discusses the rotational energy of diatomic molecules and the calculation of the rotational partition function using quantum mechanics principles. It provides equations for rotational energy and partition functions, particularly focusing on the case of a heteronuclear and homonuclear diatomic molecules, and includes examples of calculations for hydrogen (H2) at 0°C. Additionally, it relates these thermodynamic properties to entropy, free energy, and enthalpy.


![𝐶 𝑉 =
𝜕
𝜕𝑇
𝐾𝑇2
𝜕𝑙𝑛𝑄
𝜕𝑇
𝑉
𝐶 𝑉 =
𝜕
𝜕𝑇
𝐾𝑇2
×
1
𝑇
× 𝑁
𝐶 𝑉 = 𝑁𝑇
= 𝑛𝑅
Similarly,
entropy, free energy and enthalpy is related as
∴ 𝑆𝑟 = 𝑛𝑅[1 + 𝑙𝑛
8π2
IKT
ℎ2 ]
∴ 𝐺𝑟 = −𝑛𝑅𝑇𝑙𝑛
8π2
IKT
ℎ2
Hr= nRT](https://image.slidesharecdn.com/rotationalpartitionfunction-190320034358/85/Rotational-partition-function-6-320.jpg)