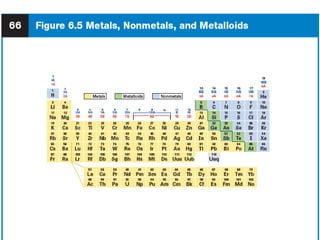
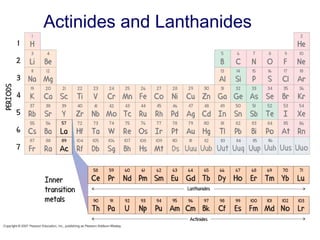
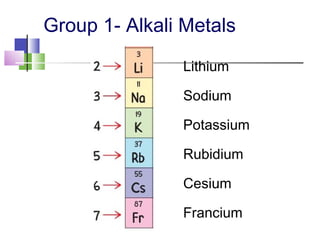
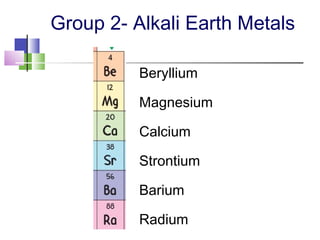
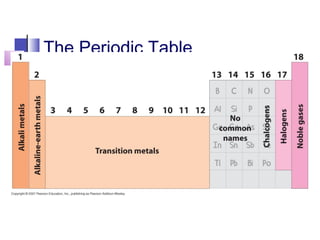
This document provides an overview of the modern periodic table. It discusses how the elements are organized by atomic number and chemical properties into periods and groups. Metals are good conductors of heat and electricity, can be hammered into sheets, and drawn into wires, while nonmetals do not share these properties. Valence electrons are the outermost electrons and influence chemical properties. Group 1 elements have 1 valence electron and form 1+ ions, with reactivity increasing down the group. Group 2 elements have 2 valence electrons and form 2+ ions, with reactivity also increasing down the group.