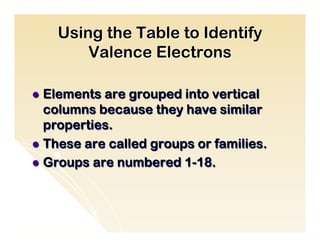
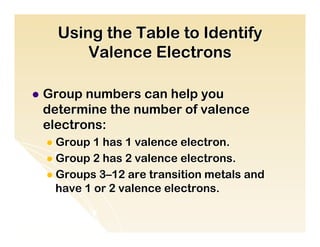
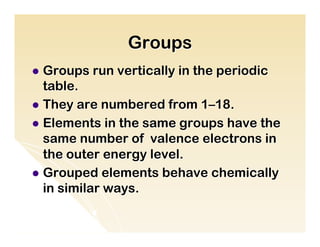
The document provides an overview of the periodic table, including its organization by atomic structure, atomic number, and chemical and physical properties. It describes the basic categories of elements as metals, nonmetals, and metalloids. Key aspects summarized include the periodic table's usefulness in predicting chemical behavior and properties of elements, how elements are grouped into periods and groups, and general characteristics of representative groups such as alkali metals, halogens, and noble gases.