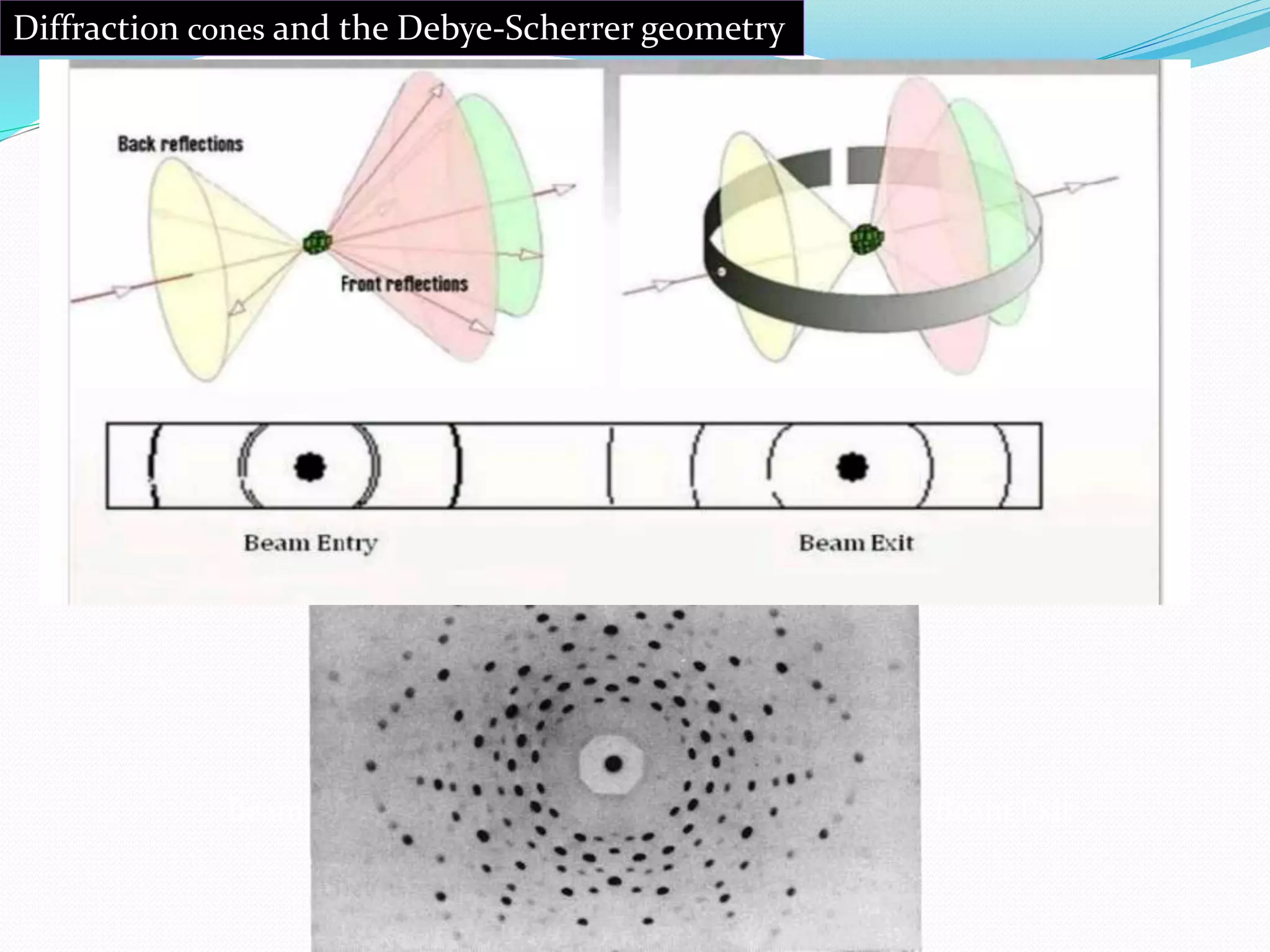
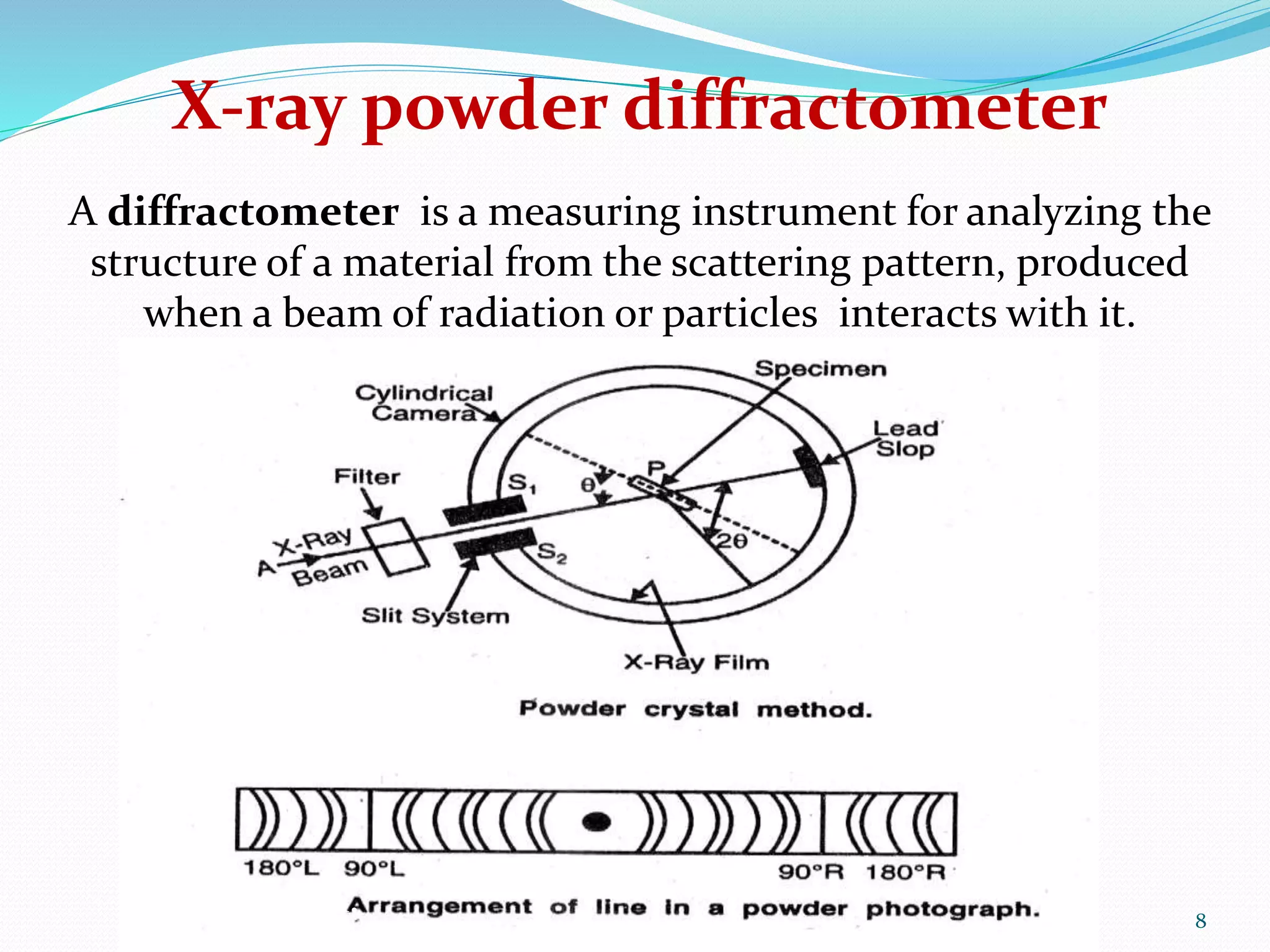
The document provides an overview of x-ray powder diffraction, including the fundamental principles of how it works, how data is obtained using an x-ray powder diffractometer, and its applications. X-ray powder diffraction utilizes x-rays and Bragg's law of diffraction to analyze the crystalline structure of materials by producing a diffraction pattern that can be used to identify unknown compounds and determine unit cell parameters. It is a powerful technique commonly used for chemical analysis and phase identification in fields such as pharmaceuticals, materials science, and mineralogy.