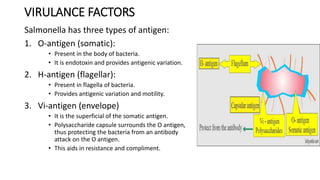
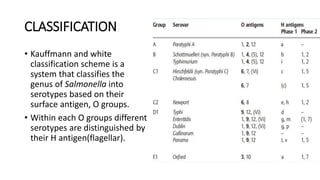
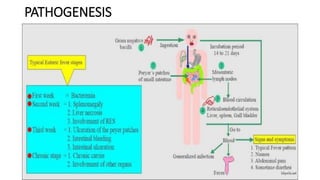
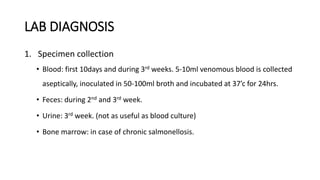
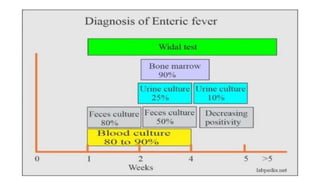
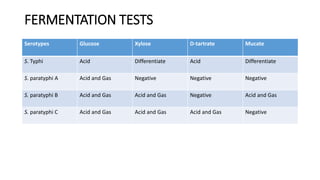
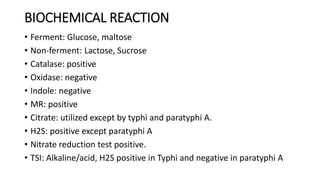
Salmonella typhi and Salmonella paratyphi cause enteric fever, also known as typhoid fever, characterized by fever, headache and abdominal discomfort. The bacteria are transmitted via contaminated food and water. Diagnosis involves blood culture early in infection or feces/urine culture later. The bacteria are identified through cultural characteristics like growth patterns and biochemical reactions. Treatment is typically with ciprofloxacin or other antibiotics. Prevention relies on sanitary food handling, clean water and vaccination.