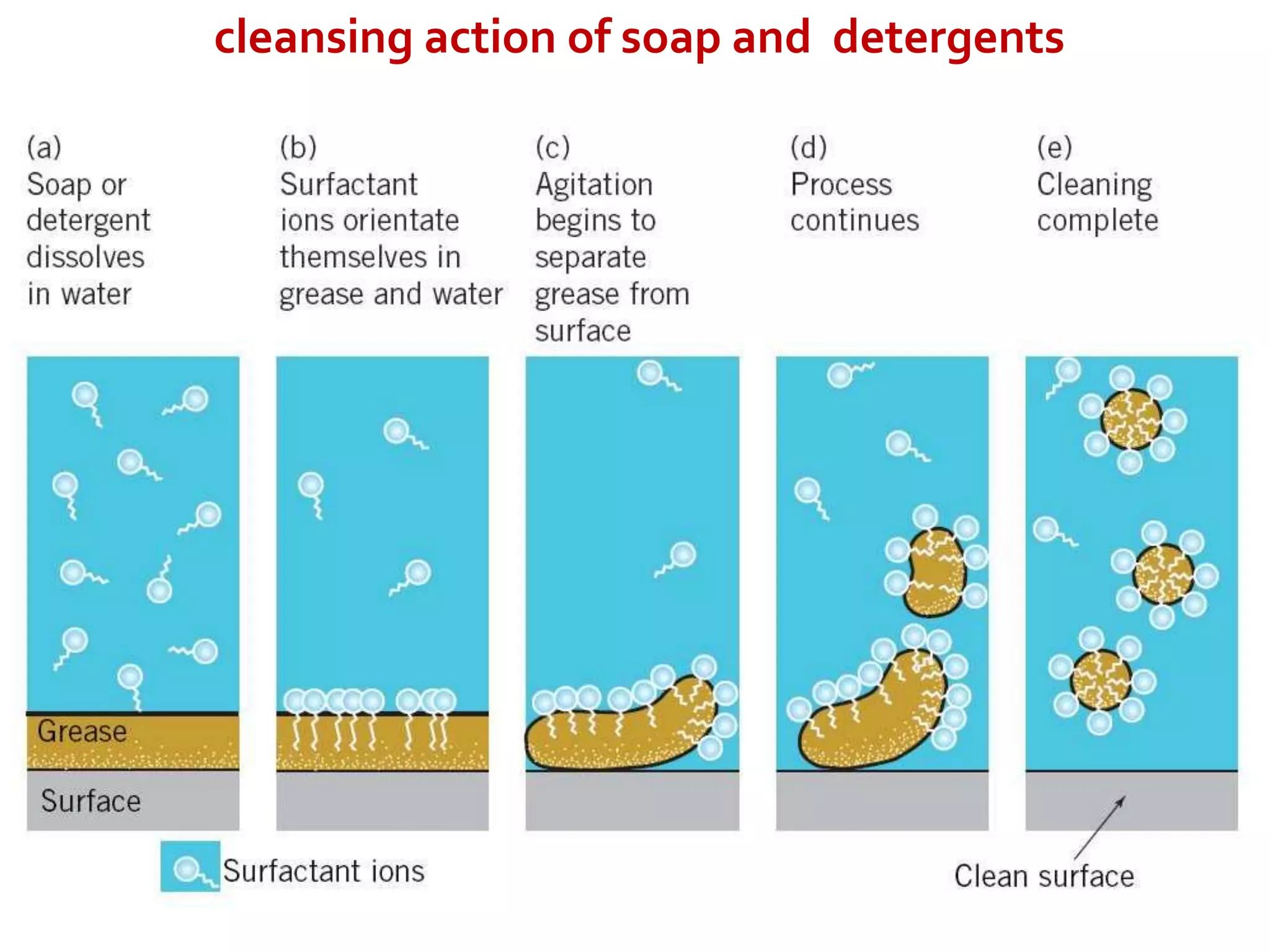
Soaps are sodium and potassium salts of long-chain carboxylic acids, made through saponification of fats with NaOH, and have eco-friendly properties but poor cleansing in hard water. Detergents, derived from petroleum, have stronger cleaning power and are effective in hard water, though they are less biodegradable and can harm aquatic life. The main distinction is that soaps create scum in hard water while detergents do not.