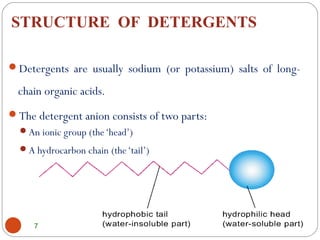
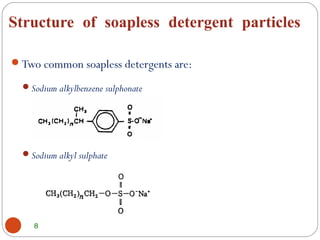
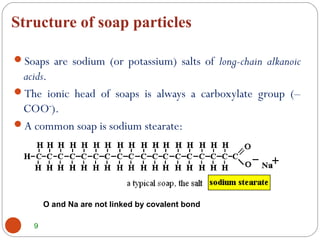
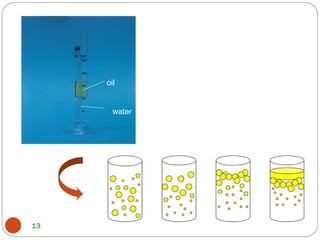
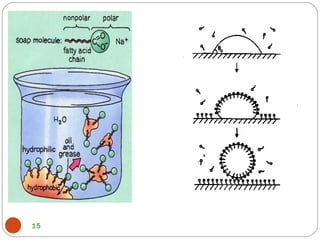
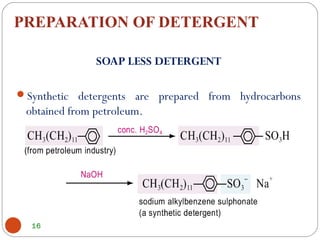
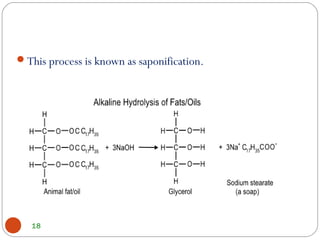
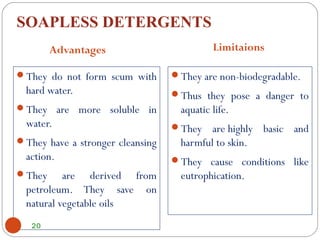
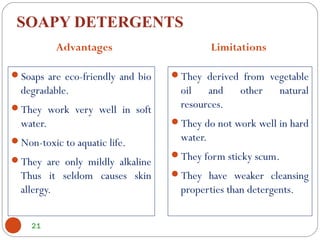
- Detergents are substances that help water cleanse things better by acting as wetting agents and emulsifying agents. There are two main types: soapless detergents made from petroleum and soaps made from animal fats or plant oils.
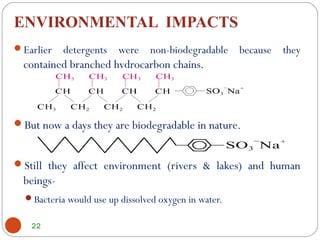
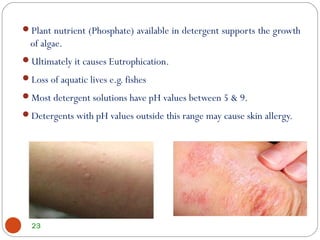
- Detergents can harm the environment by depleting oxygen levels in water and promoting algal blooms, ultimately killing aquatic life. Their use also poses risks like potential skin irritation.