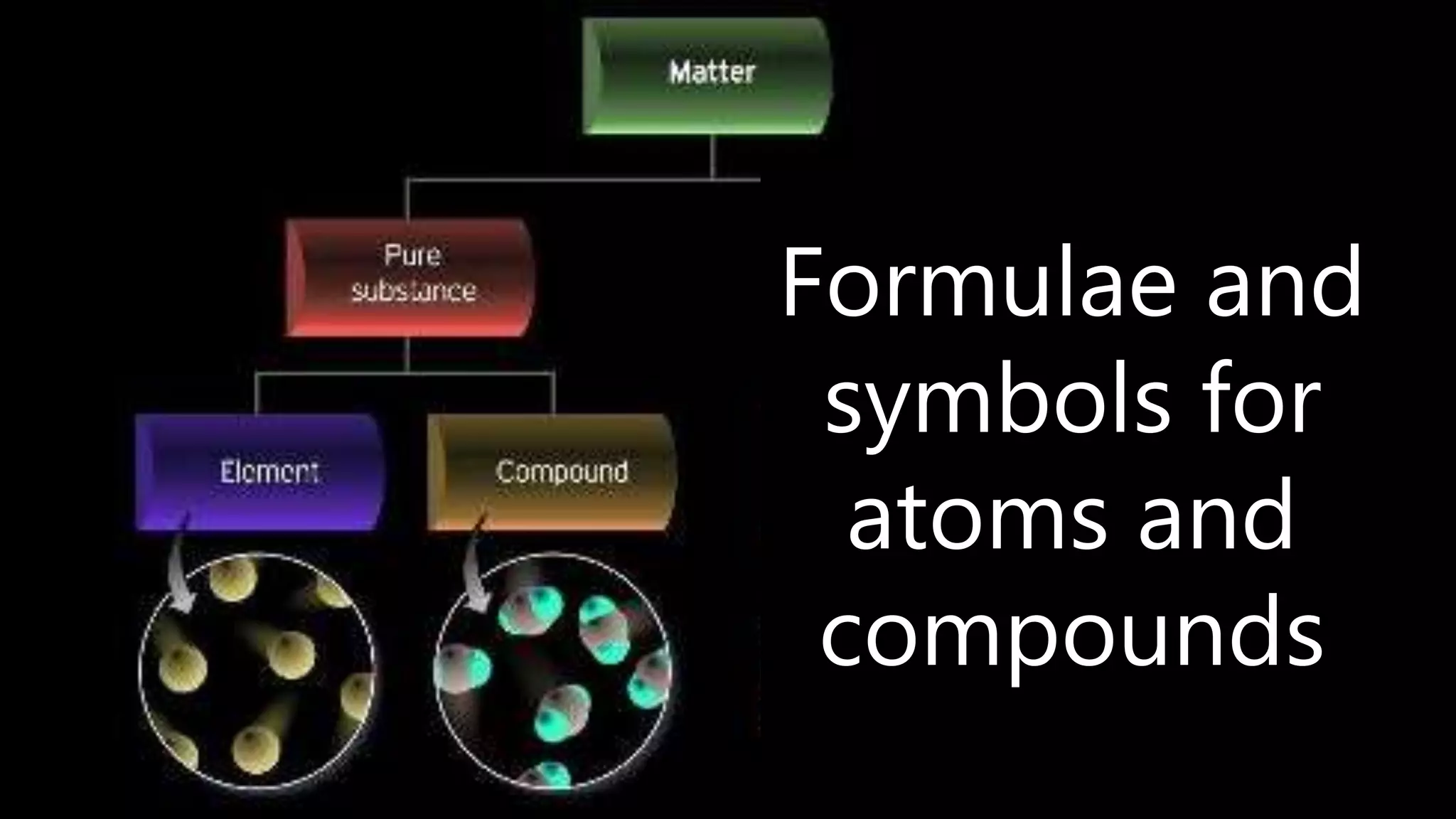
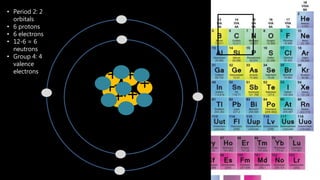
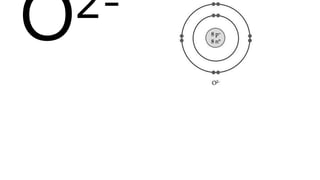
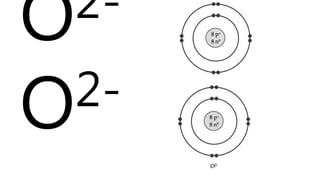
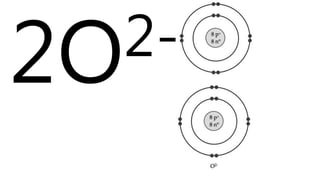
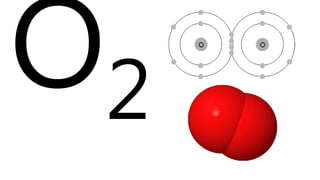
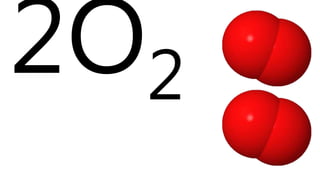
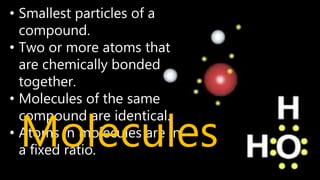
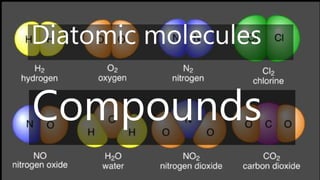
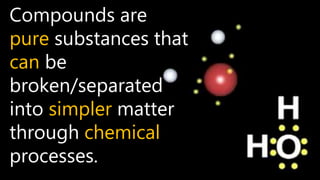
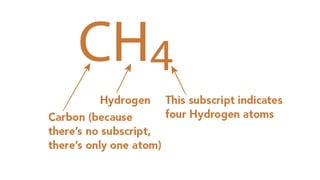
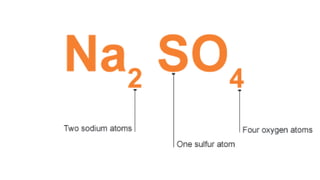
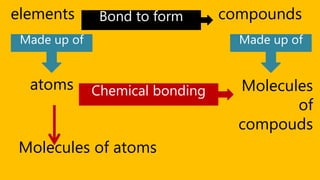
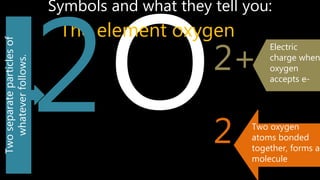Elements are the simplest and purest substances that cannot be broken down further by chemical processes. Atoms are the smallest particles that make up elements and differ in size, mass, and properties depending on the element. Molecules are formed when two or more atoms of different elements bond chemically together to form compounds. Molecules contain atoms in a fixed ratio and are the smallest particles that make up compounds, which can be broken down into simpler substances through chemical reactions. Symbols are used to represent elements, molecules, and chemical properties and reactions.