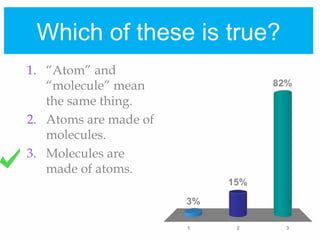
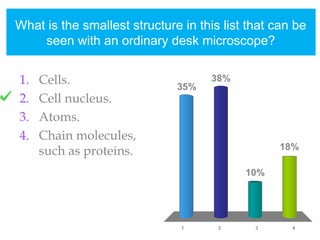
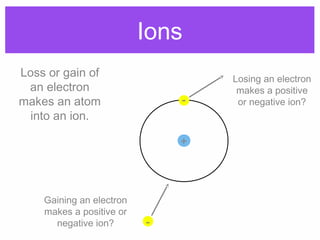
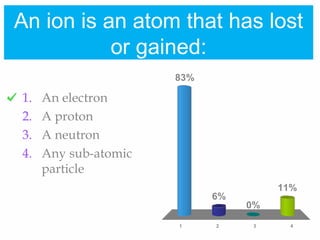
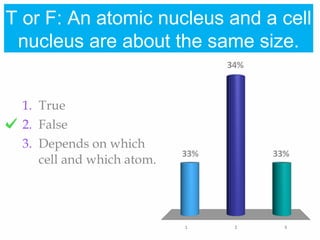
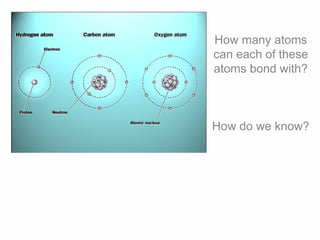
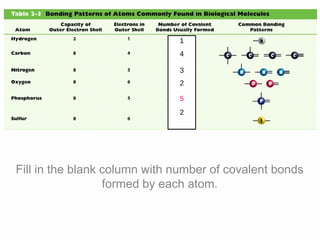
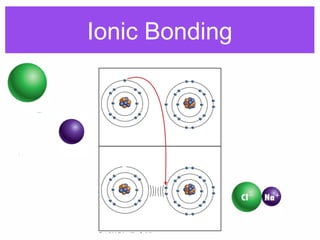
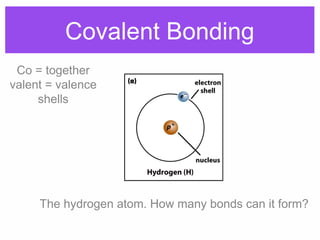
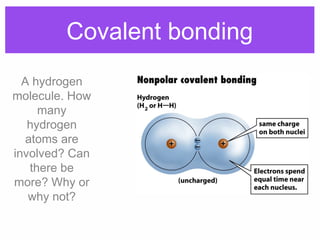
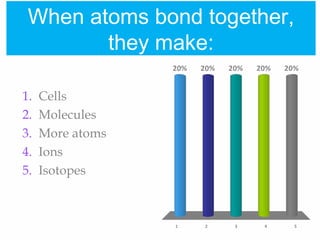
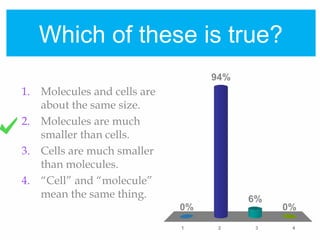
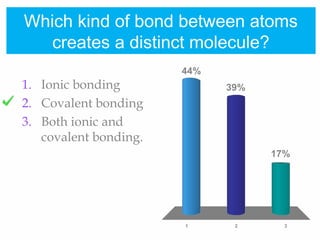
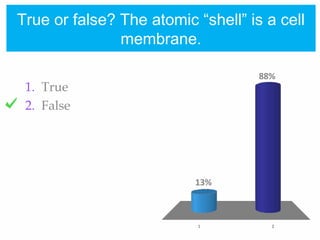
1. Atoms bond together through ionic or covalent bonding to form molecules. Ionic bonding involves the transfer of electrons between atoms, while covalent bonding involves the sharing of electrons.
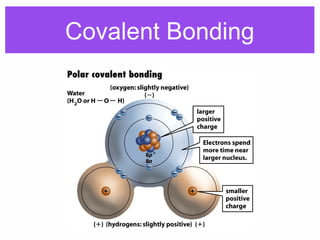
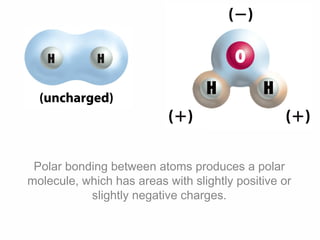
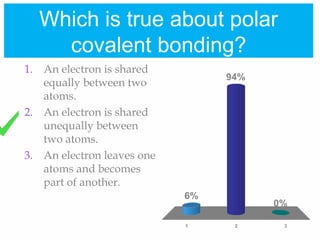
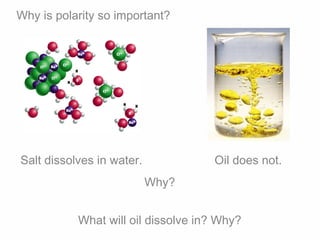
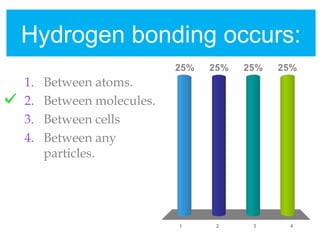
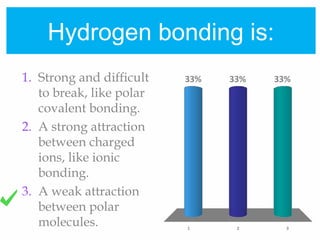
2. Polar covalent bonding results in a molecule with slight positive and negative regions due to the unequal sharing of electrons. Hydrogen bonding, a weak attraction between polar molecules like water, is important for properties such as water's high boiling point and ability to dissolve many other substances.
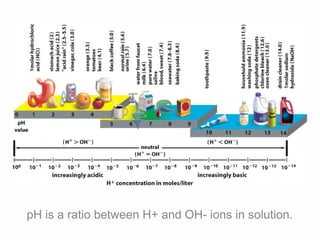
3. The pH scale measures how many hydrogen ions (H+) and hydroxide ions (OH-) are present in a solution, indicating its acidity or alkalinity. Polarity allows water molecules to separate and surround ions, affecting