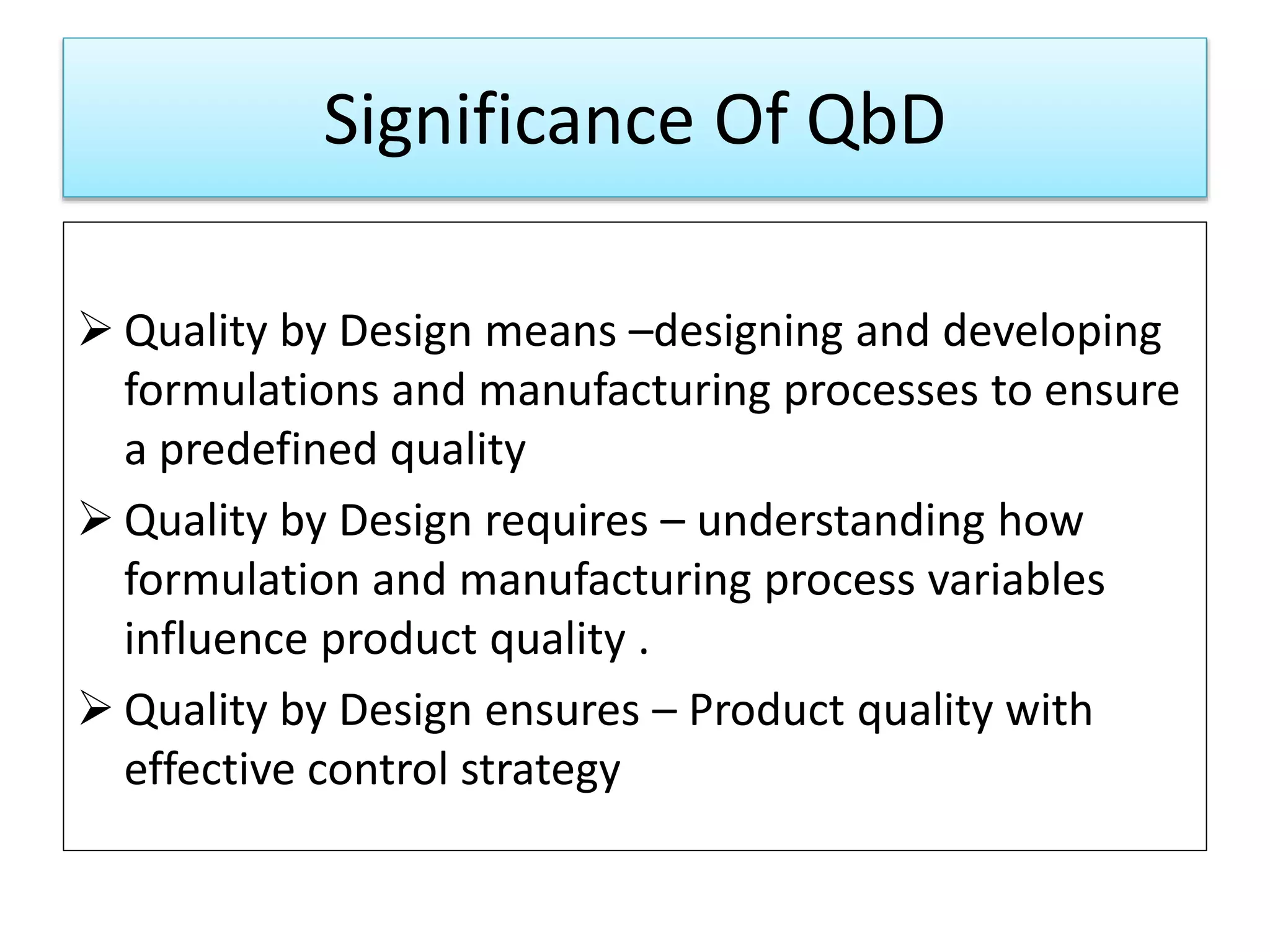
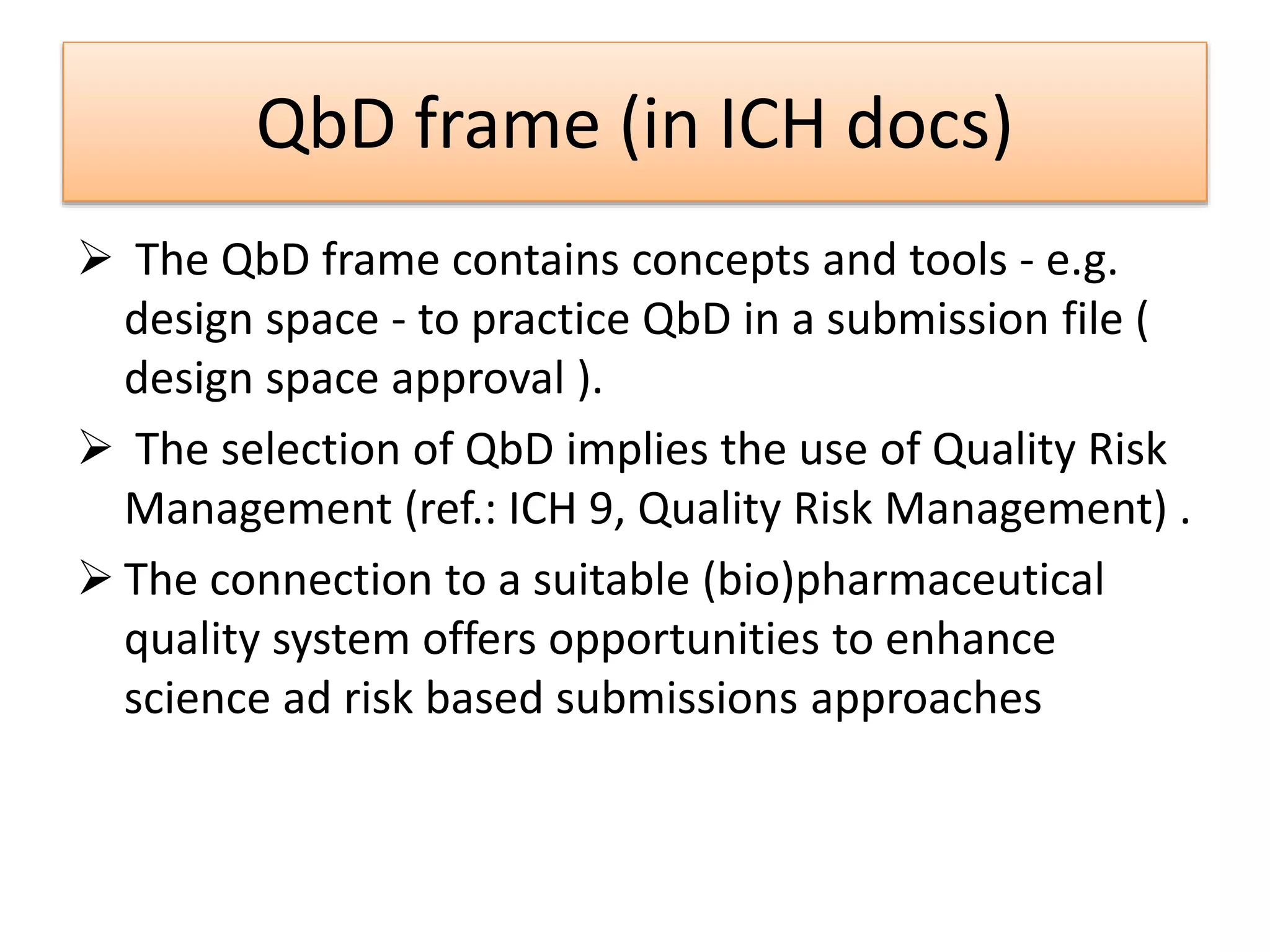
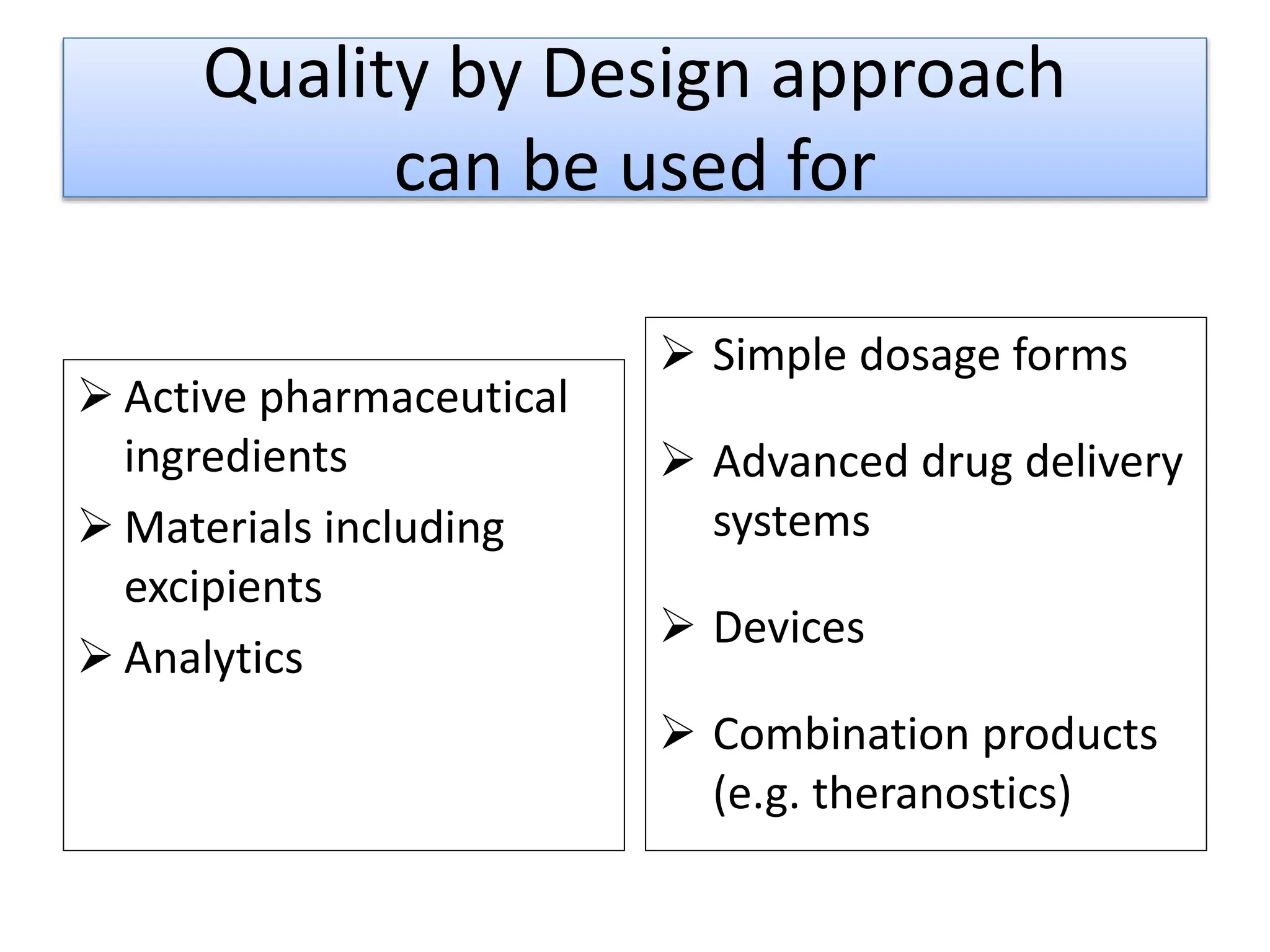
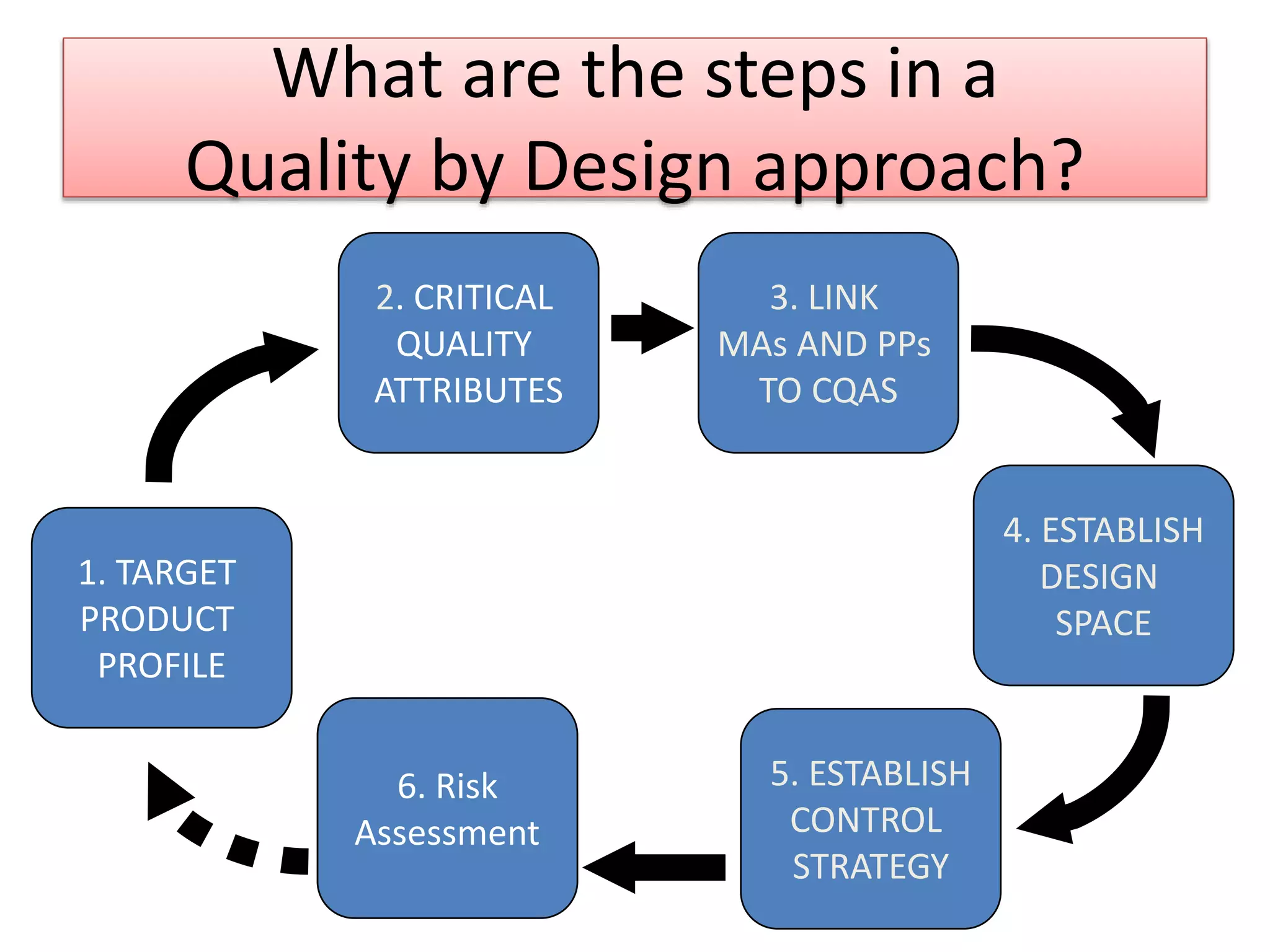
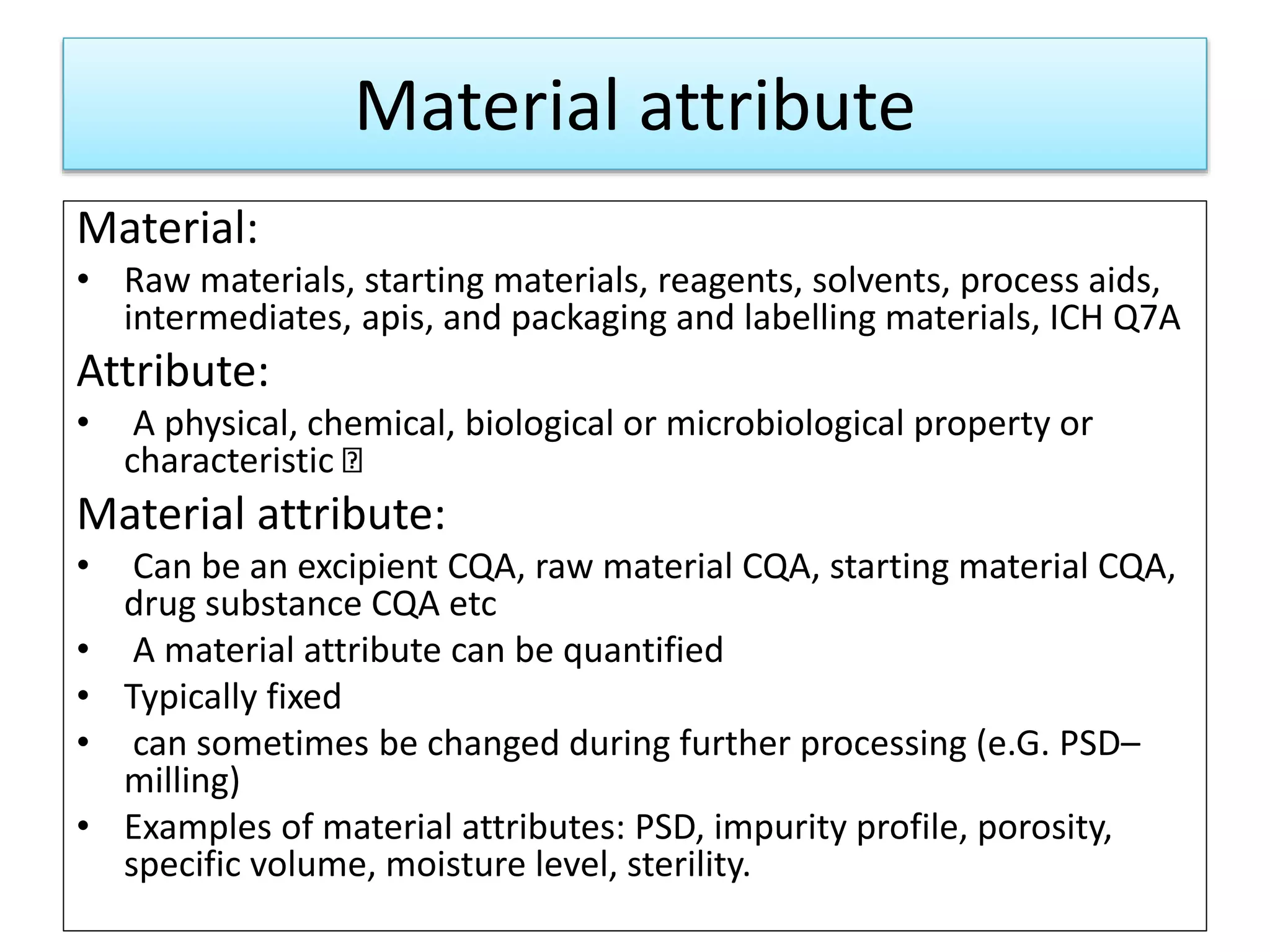
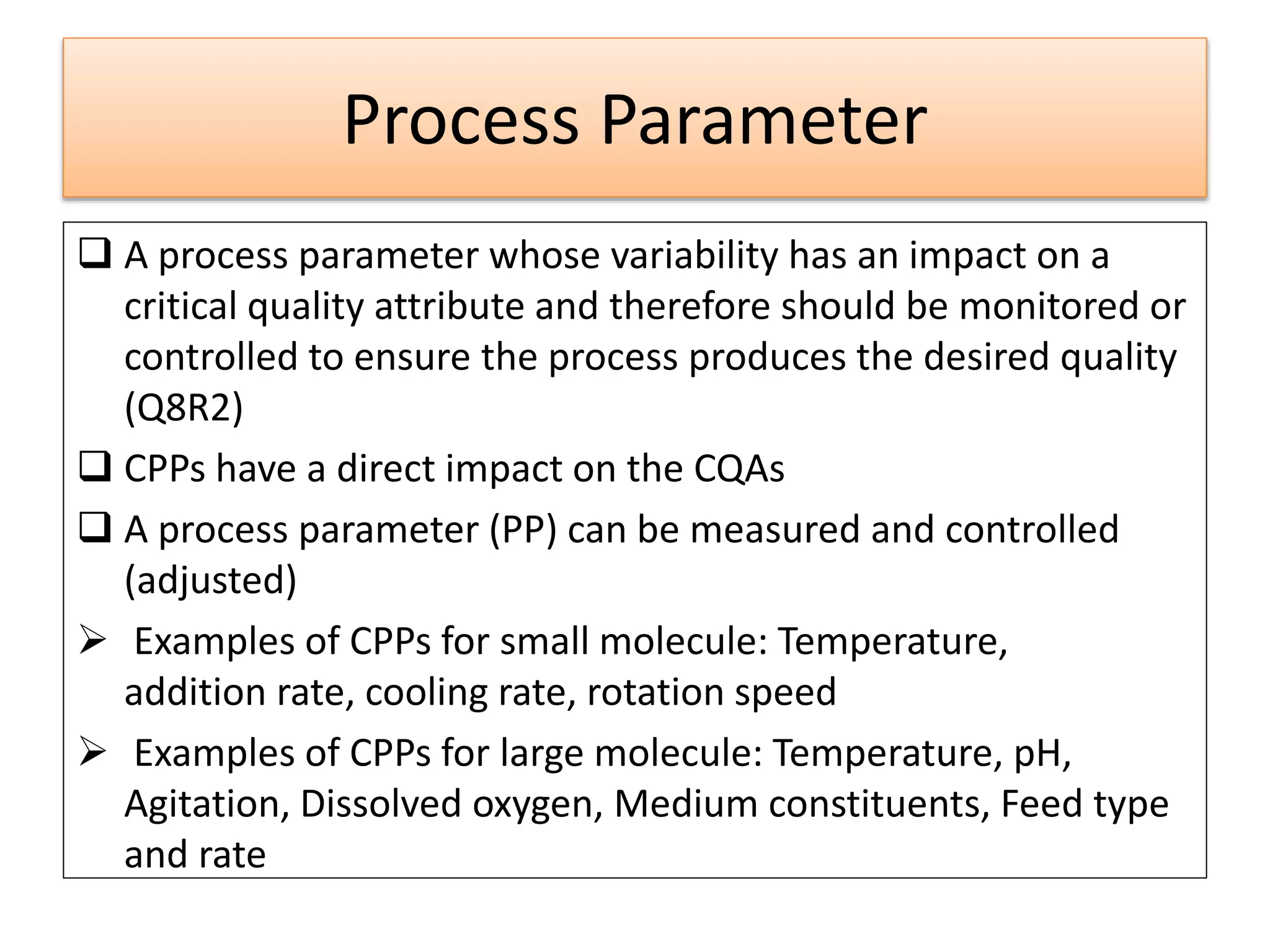
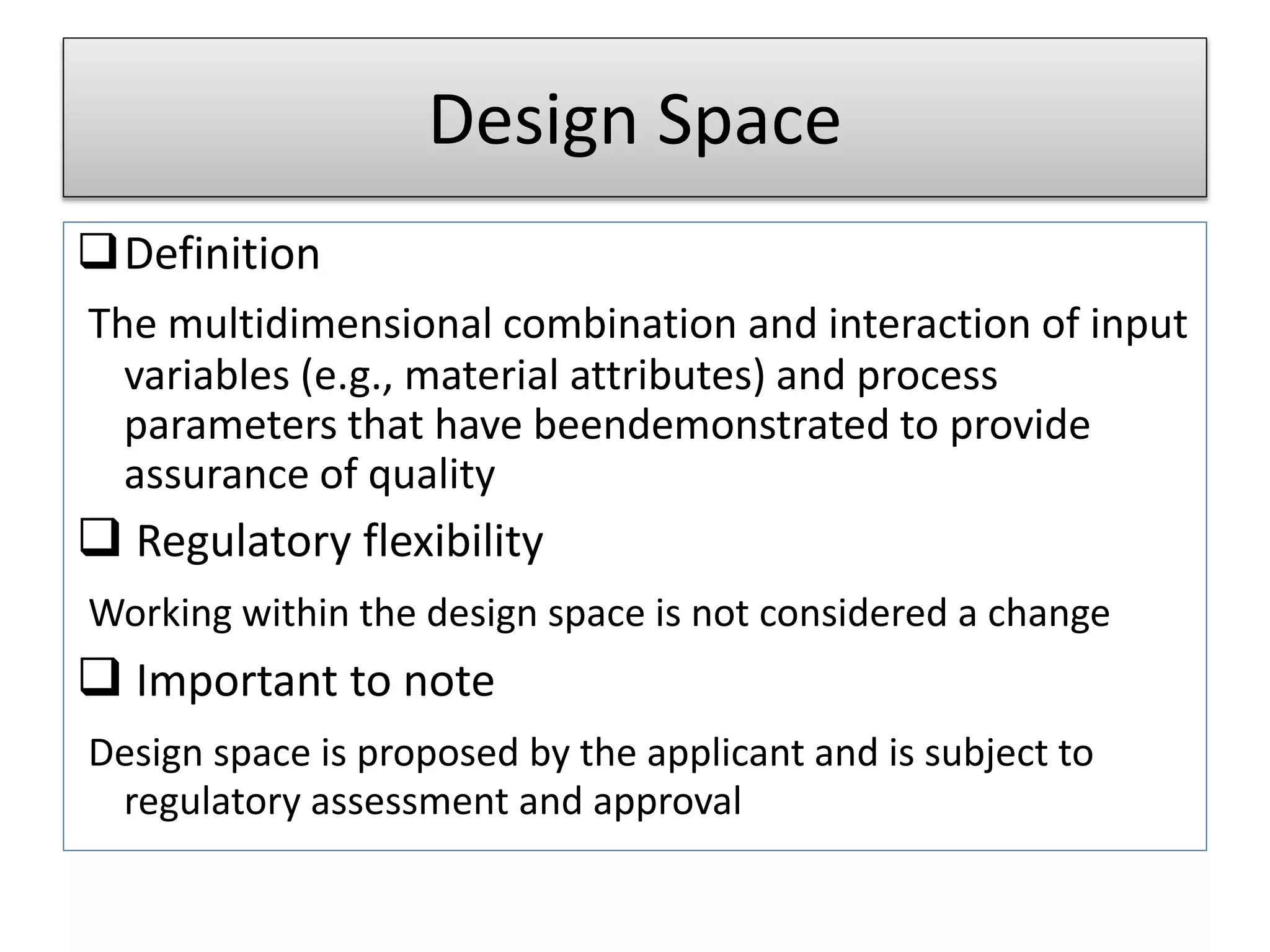
This presentation provides an overview of Quality by Design (QbD), a systematic approach to pharmaceutical development that begins with predefined product quality objectives. The key aspects of QbD include establishing a Target Product Profile, identifying Critical Quality Attributes, determining material attributes and critical process parameters linked to Critical Quality Attributes, defining a design space, establishing a control strategy, and conducting risk assessments. QbD aims to ensure final drug product quality through understanding manufacturing processes and controls.










![References
• [1] ICH Guideline Q8 – Pharmaceutical Development,
http://www.ich.org (10 Nov 2005).
• [2] U.S. Food and Drug Administration Guidance for Industry. PAT –
A Framework for Innovative.
• [3] J.C. Berridge An Update on ICH Guideline Q8 – Pharmaceutical
Development, www.fda.gov/ohrms/dockets/AC/06/ slides/2006-
4241s1_2.ppt, ISPE Vienna Congress 2006.](https://image.slidesharecdn.com/qualitybydesignqbd-150327102537-conversion-gate01/75/Quality-by-design-QbD-19-2048.jpg)