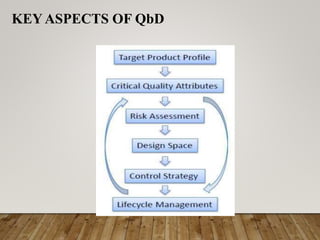
This document discusses quality by design (QbD), which is a systematic approach to pharmaceutical development that begins with predefined objectives and emphasizes product and process understanding based on sound science and quality risk management. It involves designing and developing formulations and manufacturing processes to ensure a predefined quality. The key aspects of QbD include active pharmaceutical ingredients, excipients, analytics, simple and advanced dosage forms, devices, and combination products. The main steps in the QbD approach are defining the target product profile, critical quality attributes, material and process parameters, design space, control strategy, and risk management.