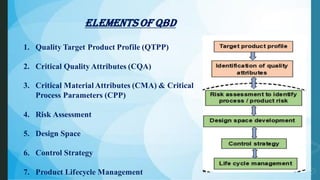
This document provides an overview of Quality by Design (QBD), a systematic approach for developing quality pharmaceutical products. It discusses key QBD elements like quality target product profiles, critical quality attributes, critical material attributes, risk assessment, design space, and control strategy. The document also outlines tools used in QBD like design of experiments and process analytical technology. It highlights advantages of QBD such as improved product quality assurance and opportunities for continual improvement. Finally, it discusses various applications of QBD in areas like chromatography, spectroscopy, biopharmaceuticals, and more.