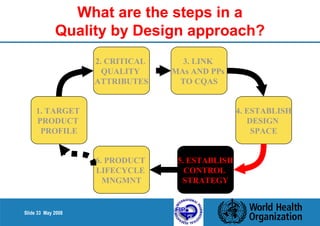
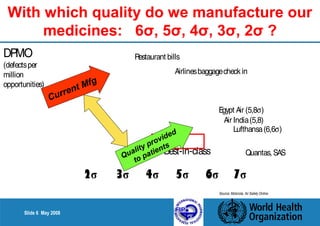
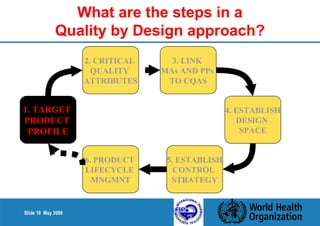
This document summarizes the steps in a Quality by Design (QbD) approach for pharmaceutical development:
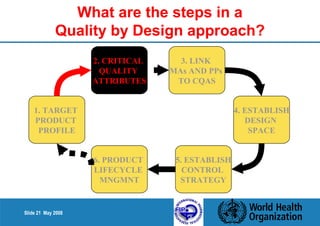
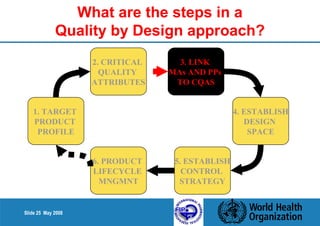
1. Define the Target Product Profile to outline the desired quality characteristics.
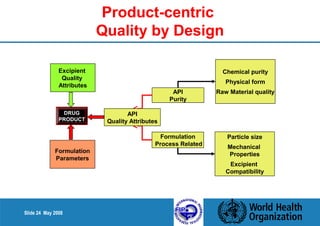
2. Determine the Critical Quality Attributes that ensure the product's safety, efficacy and quality.
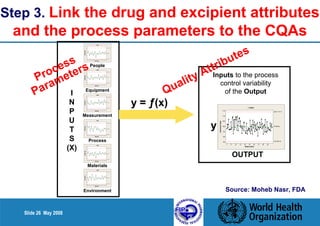
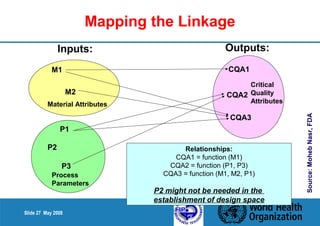
3. Link the material attributes, process parameters and Critical Quality Attributes through experimental studies and risk assessment.
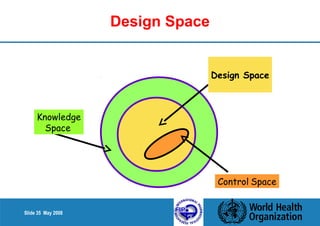
4. Define the Design Space as the multidimensional combination of input variables and process parameters that provide quality assurance.
5. Establish a control strategy for inputs, processes and outputs to maintain final product quality within the design space.




















![Slide 32 May 2008
Definition of Design Space
The material attributes and process parameters that
assure quality.
The multidimensional combination
and interaction of input variables
(e.g. material attributes) and
process parameters that have been
demonstrated to provide assurance
of quality.
Roll pressure
Gap width
Screen Size
300
200
100
0
-100
Dataset - Run1-10a.M3
Observed vs. Predicted $Time [Last comp.] (Aligned)
0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 210 220 230 240 250 260
$Time (normalized)
SIMCA-P+ 11.5 - 05/02/2007 23:17:07](https://image.slidesharecdn.com/qualitydesign-140920223401-phpapp01/85/Quality-design-32-320.jpg)