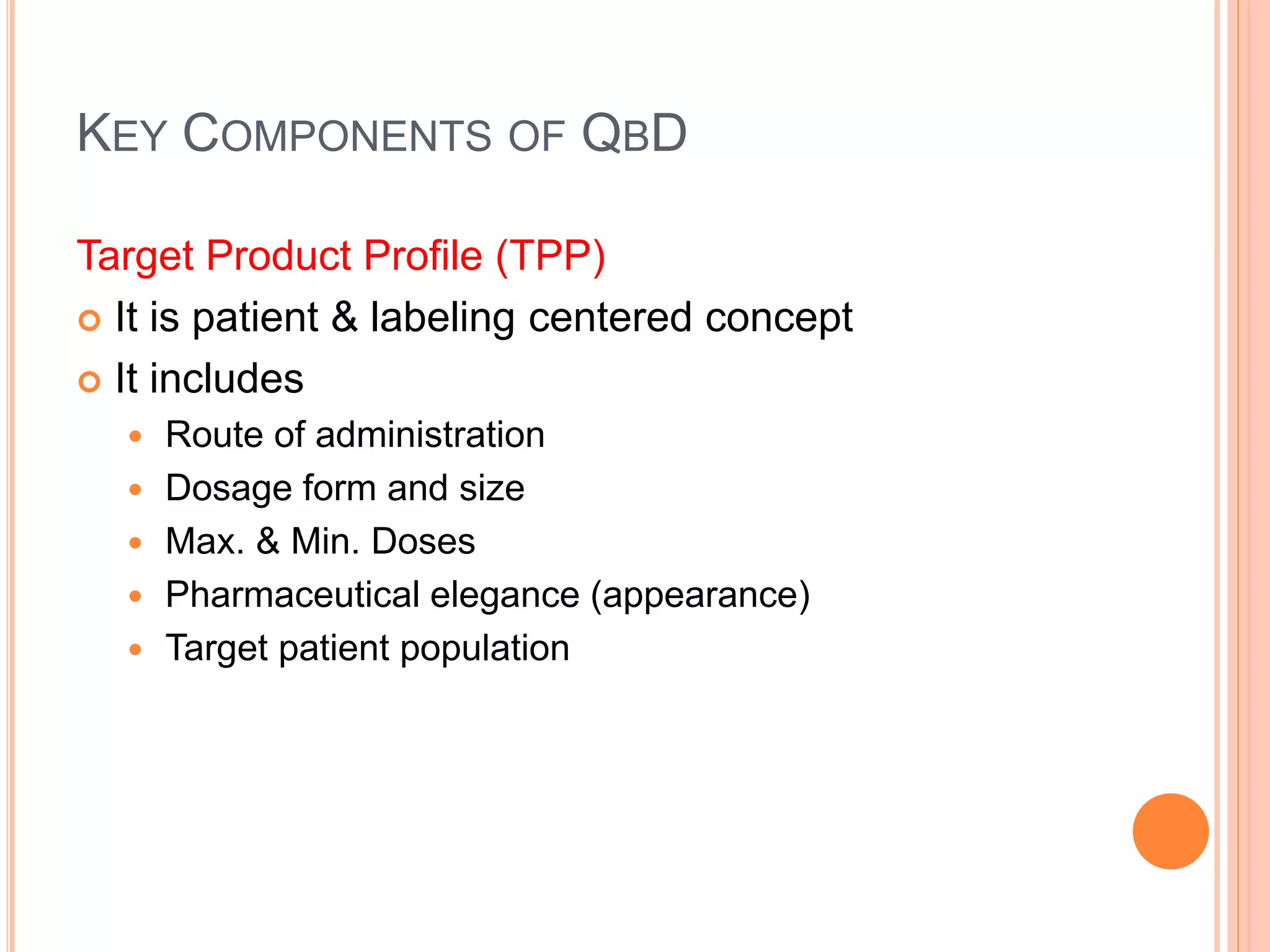
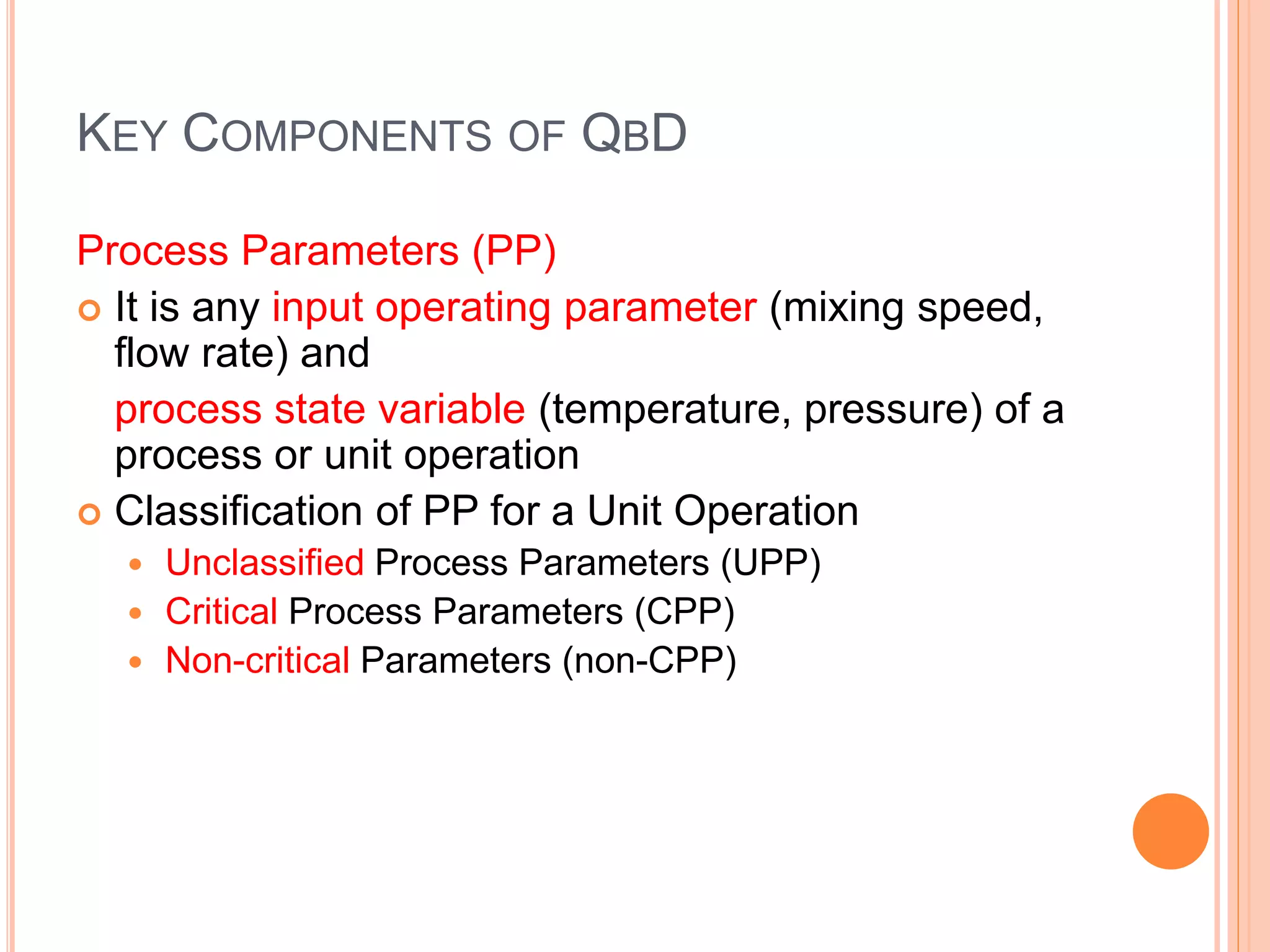
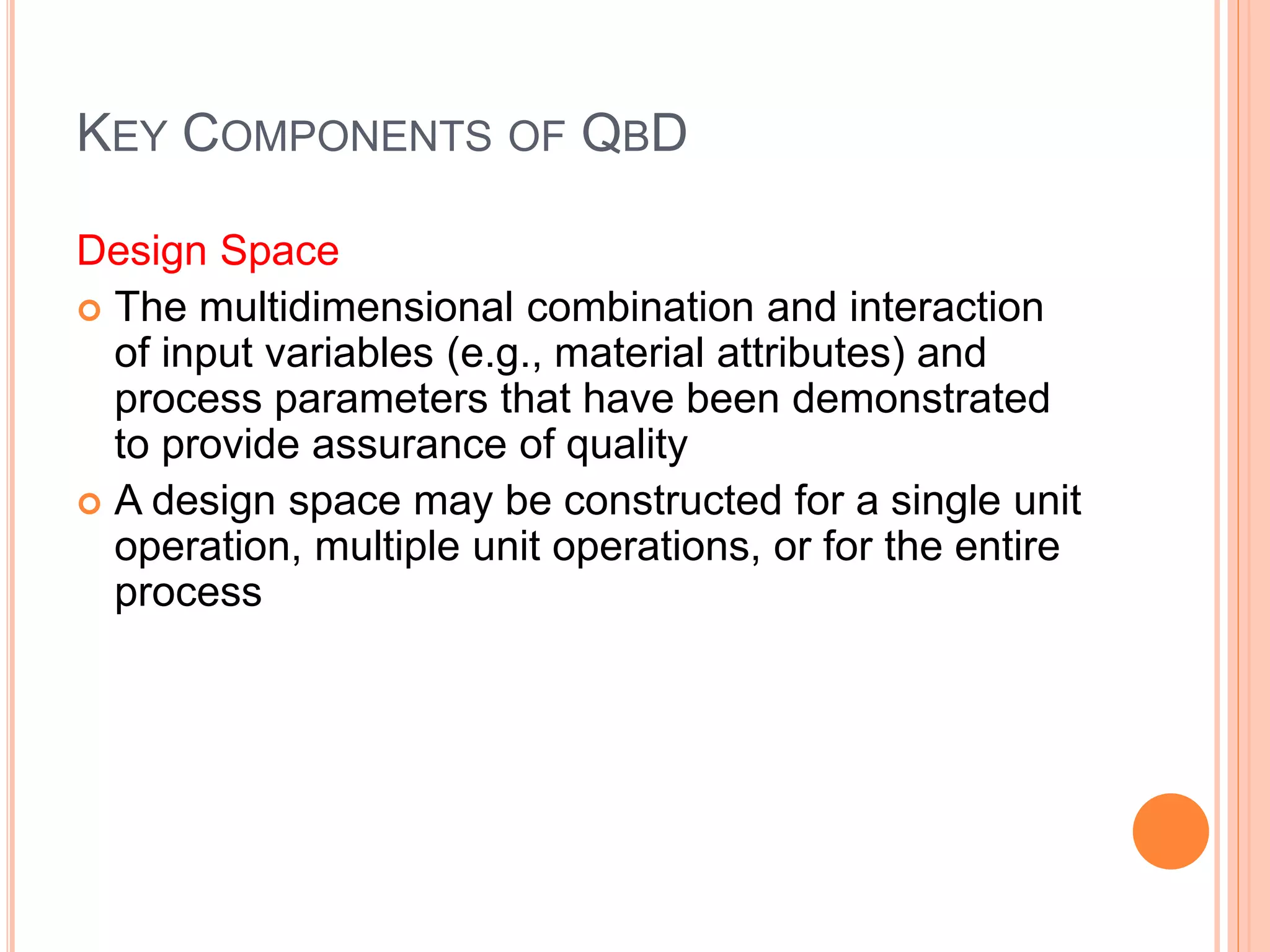
The document introduces Pharmaceutical Quality by Design (QbD), emphasizing a systematic approach to enhance product and process understanding in pharmaceutical development, moving away from traditional testing methods. It outlines the benefits for both manufacturers and regulators, such as improved product quality, reduced time to market, and increased innovation flexibility. Key components of QbD include target product profiles, critical quality attributes, and process parameters, all aimed at ensuring quality through proactive design and risk management.