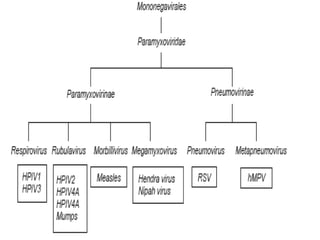
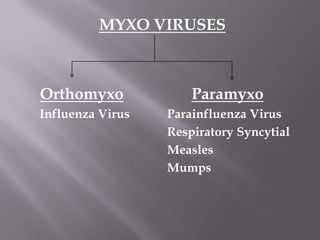
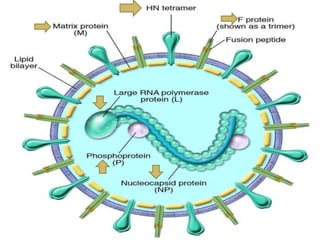
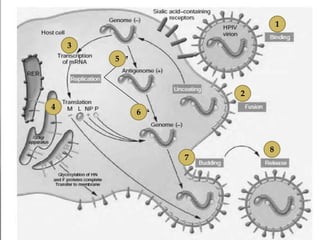
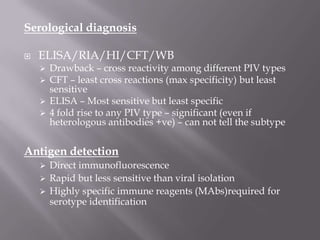
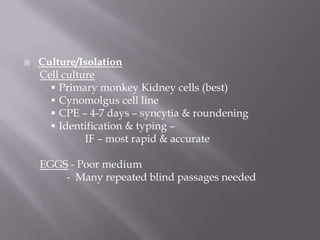
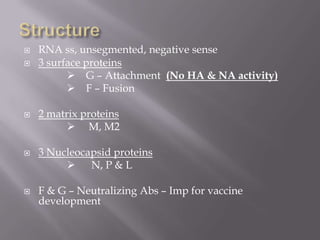
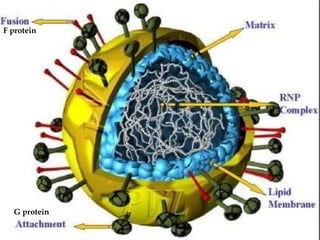
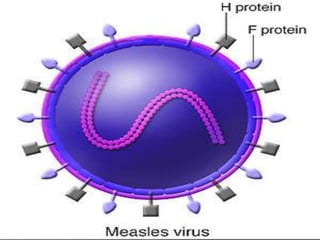
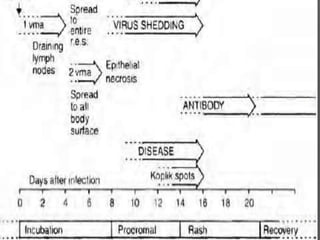
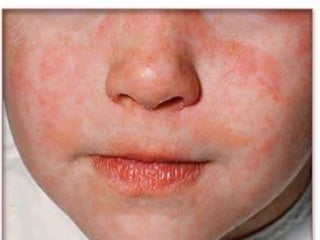
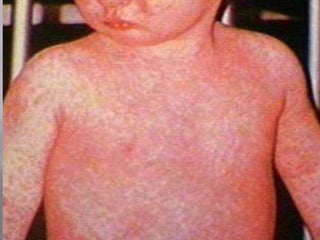
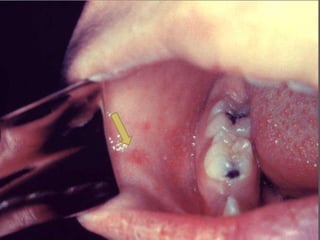
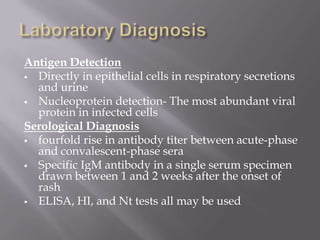
The document discusses Myxoviruses, which are RNA viruses that infect the respiratory mucosa. It specifically focuses on Orthomyxoviruses like influenza virus and Paramyxoviruses such as parainfluenza virus, respiratory syncytial virus, measles virus, and mumps virus. It provides details on the structure, transmission, pathogenesis, diagnosis, and treatment of these important respiratory viruses.