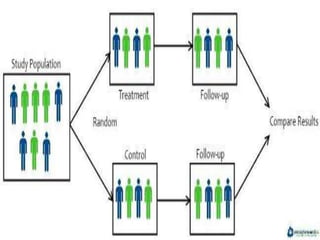
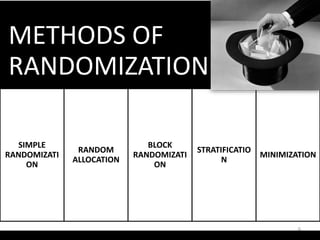
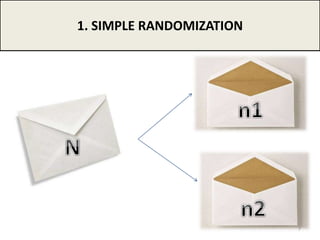
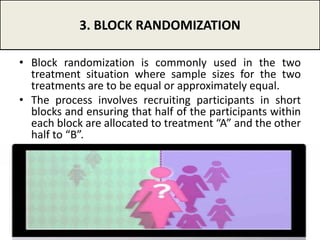
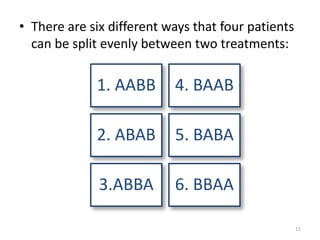
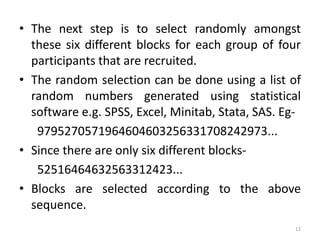
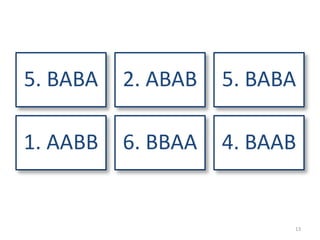
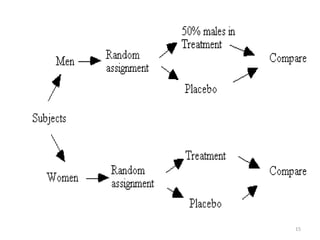
The document provides an overview of randomization methods used in clinical trials, emphasizing the importance of unbiased treatment group allocation and comparability. It details various randomization techniques including simple randomization, random allocation, block randomization, stratification, and minimization. Additionally, the document discusses considerations for sample size, data collection, and monitoring management to ensure the integrity of clinical trial results.