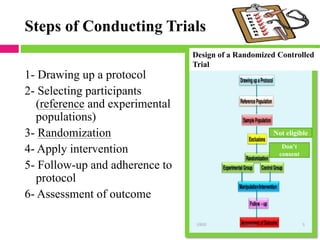
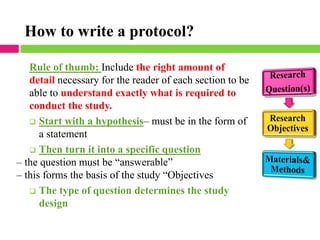
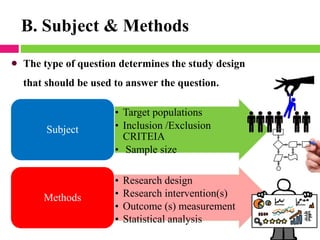
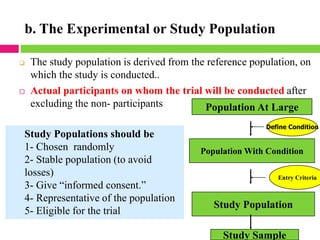
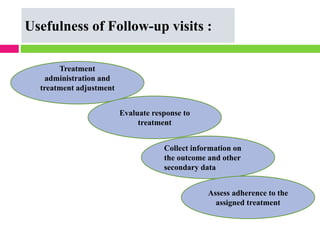
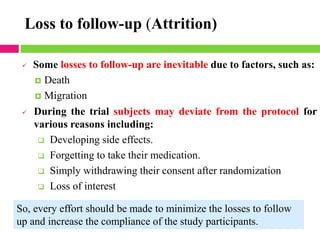
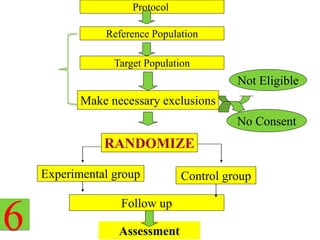
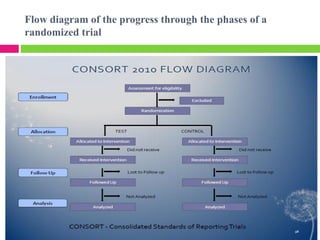
This document outlines the key steps in conducting a clinical trial:
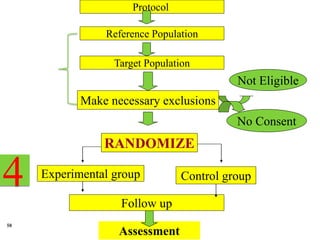
1. Drawing up a detailed research protocol that serves as the trial's operating manual.
2. Selecting and screening participants according to eligibility criteria to identify the study population. Sample size is also calculated.
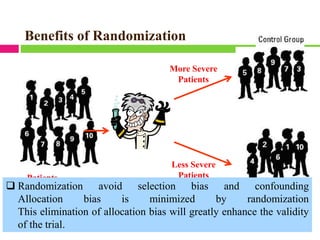
3. Randomly allocating the study participants into experimental and control groups through a process like randomization to reduce bias.