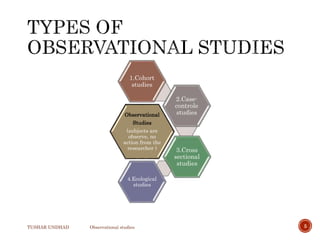
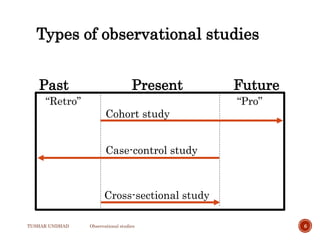
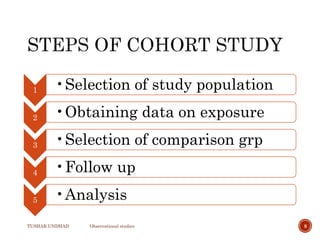
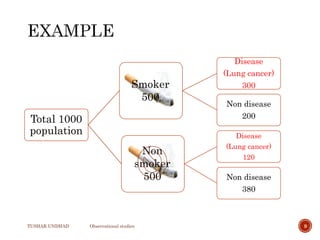
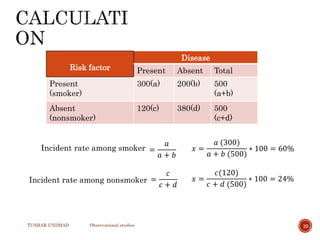
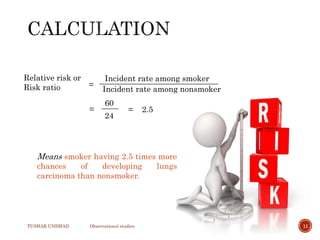
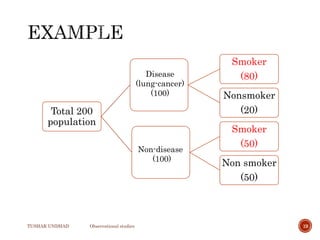
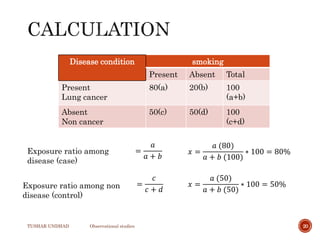
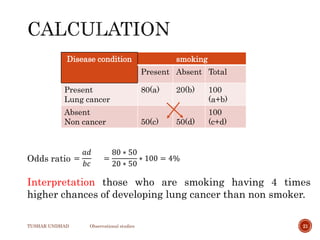
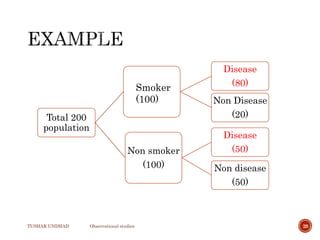
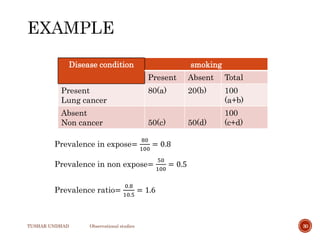
The document provides a comprehensive overview of observational studies in pharmacology, detailing their definition, advantages, and disadvantages. It discusses different types of observational studies including cohort, case-control, and cross-sectional studies, along with methodologies and applications. It highlights the practical implications of these studies, including their use in evaluating disease outcomes and risk factors, while also noting challenges like selection bias and the need for large populations.