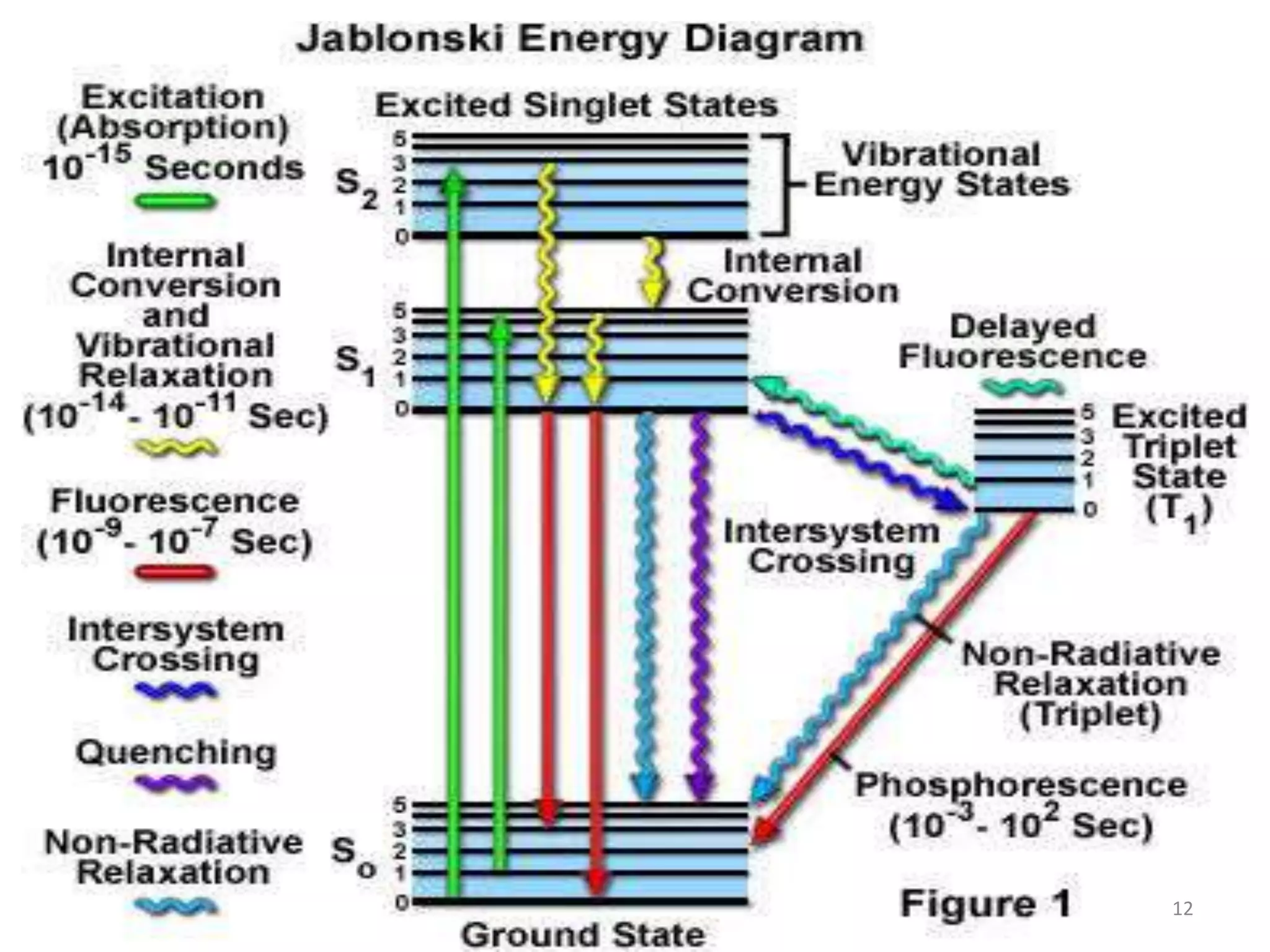
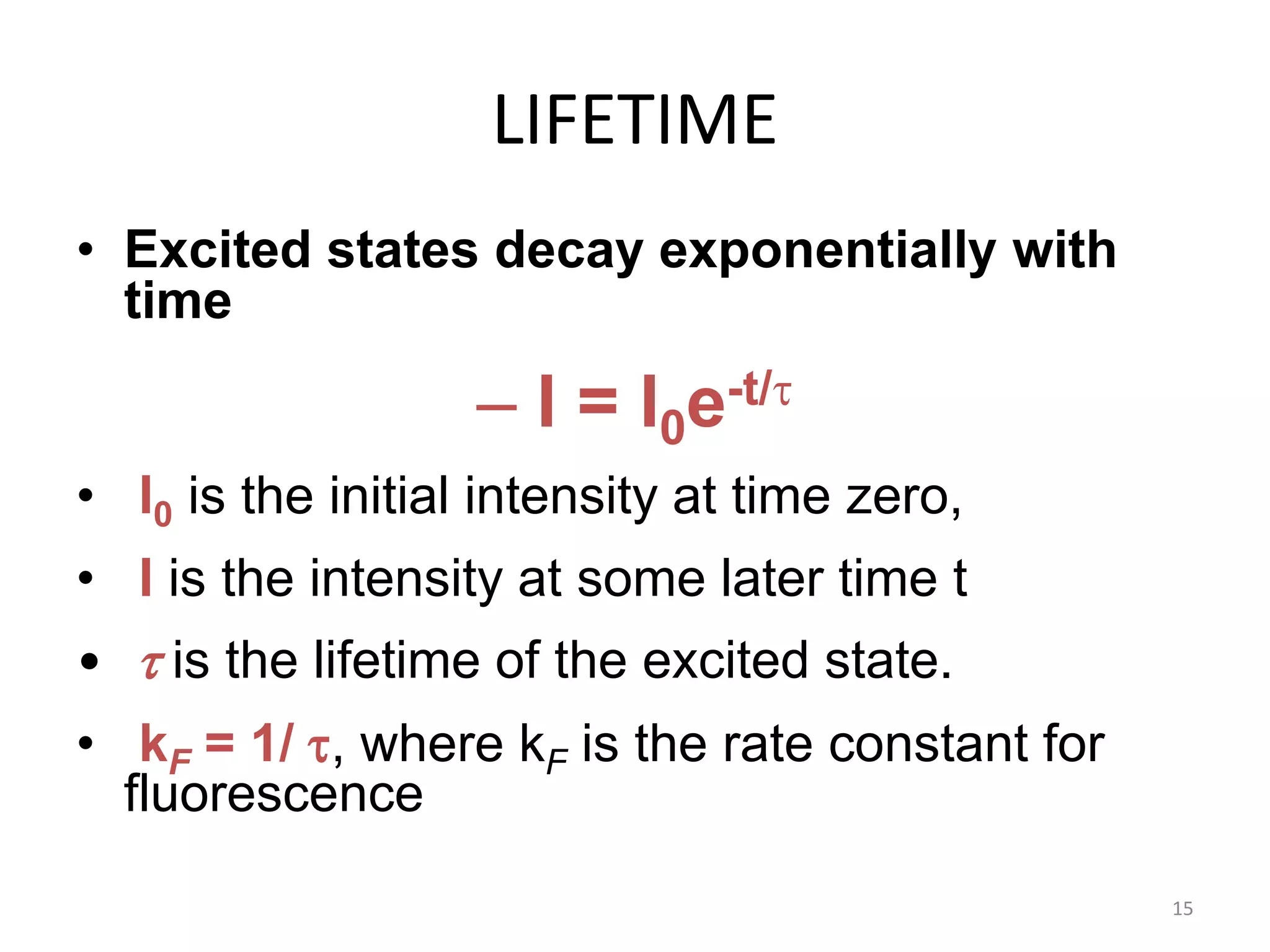
The document discusses the theory of fluorimetry. It begins by defining luminescence as light emission from a substance when an electron returns to the ground state from an excited state. It then describes the three types of luminescence - fluorescence, phosphorescence, and chemiluminescence. Fluorescence occurs immediately when light is absorbed, while phosphorescence occurs more slowly after light is removed. Fluorimetry is the measurement of fluorescence, involving excitation and emission spectra. The document goes on to discuss singlet and triplet electronic states, Stokes shift, lifetime, quantum yield, and references in the field of fluorimetry.