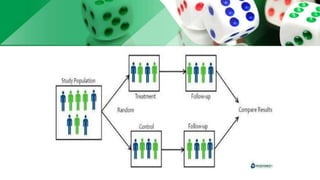
This document discusses various methods of randomization used in clinical trials. It begins by describing the purpose of randomization as removing bias and achieving comparability between treatment groups. It then explains different randomization methods like simple randomization, random allocation, block randomization, and stratification. Block randomization randomly assigns participants in blocks to ensure equal allocation. Stratification divides participants according to prognostic factors. The document also discusses sample size determination, documentation, and monitoring in clinical trials.