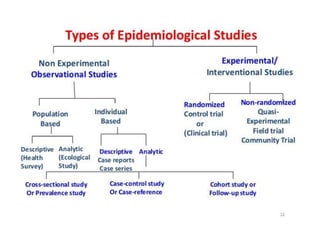
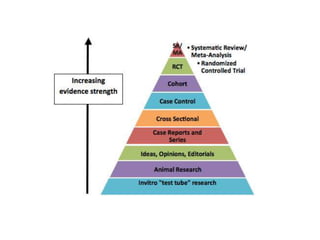
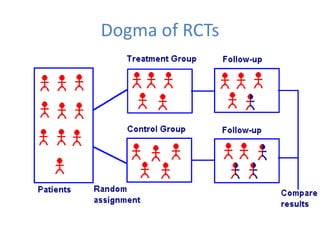
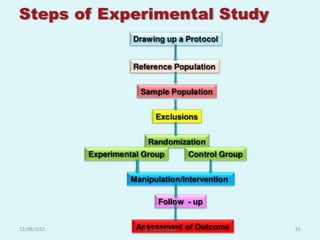
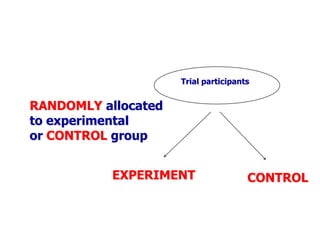
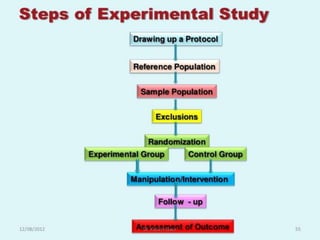
- Randomized controlled trials (RCTs) are experiments in which people are randomly allocated to different intervention groups in order to evaluate the effects of those interventions.
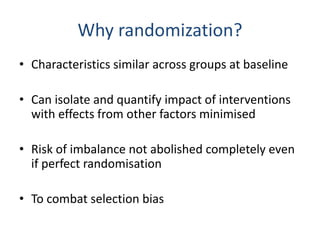
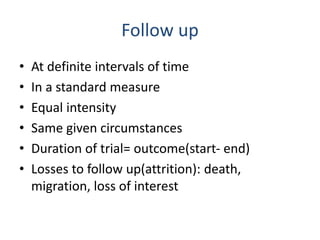
- RCTs help reduce bias and allow for comparisons between groups that are otherwise similar. Random allocation means each participant has an equal chance of being placed in any group.
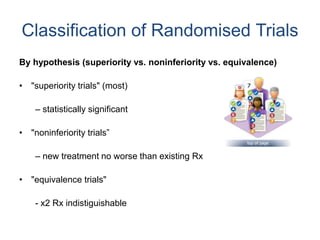
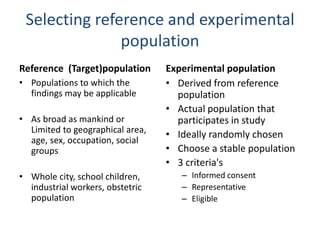
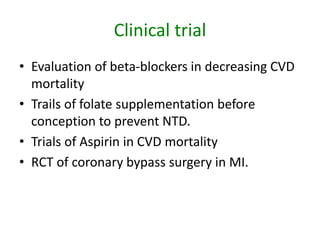
- RCTs involve an experimental group that receives the new intervention being tested and a control group that receives an alternative treatment, no treatment, or a placebo. Comparing outcomes between the groups allows researchers to determine the efficacy of the intervention.