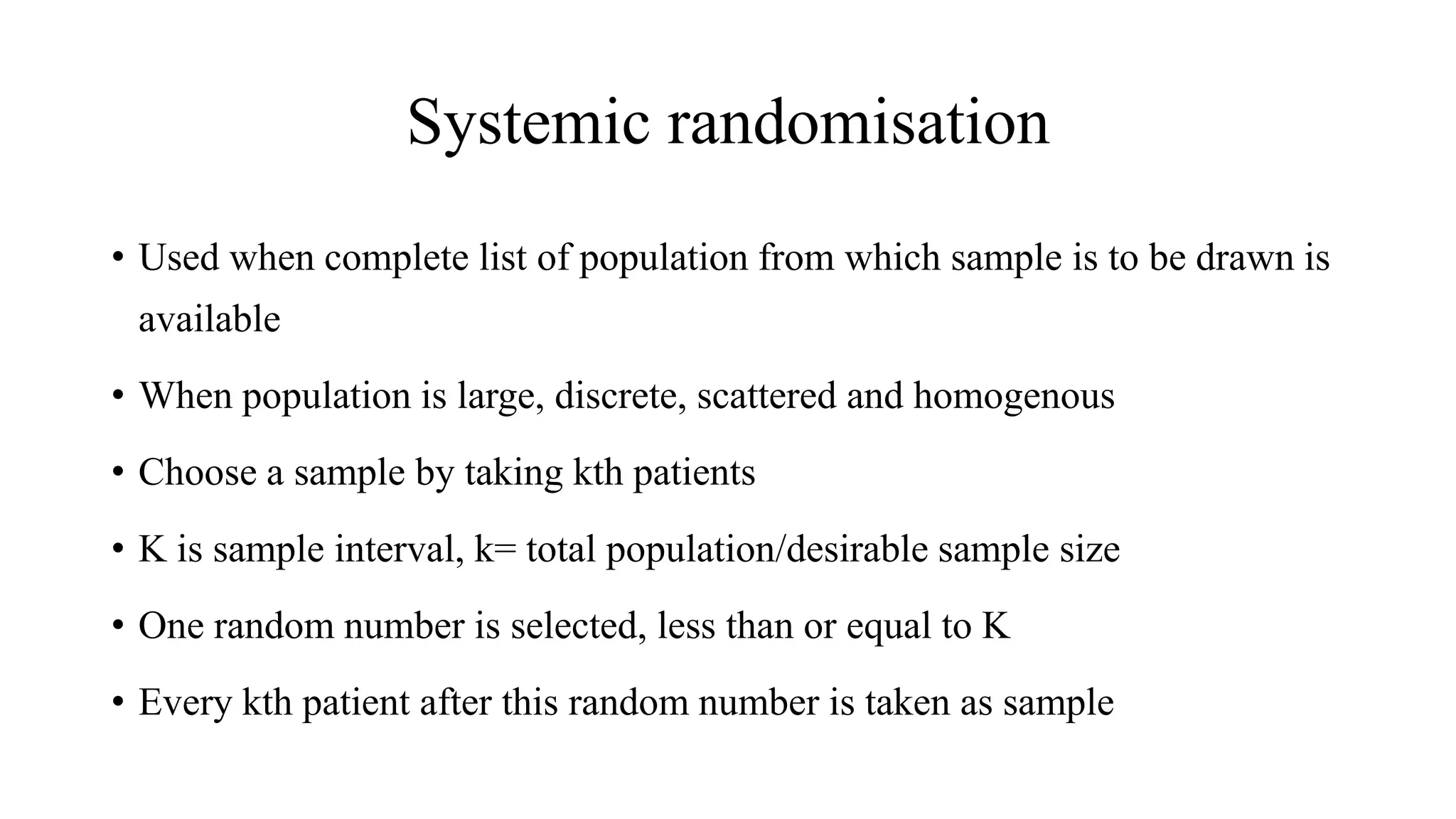
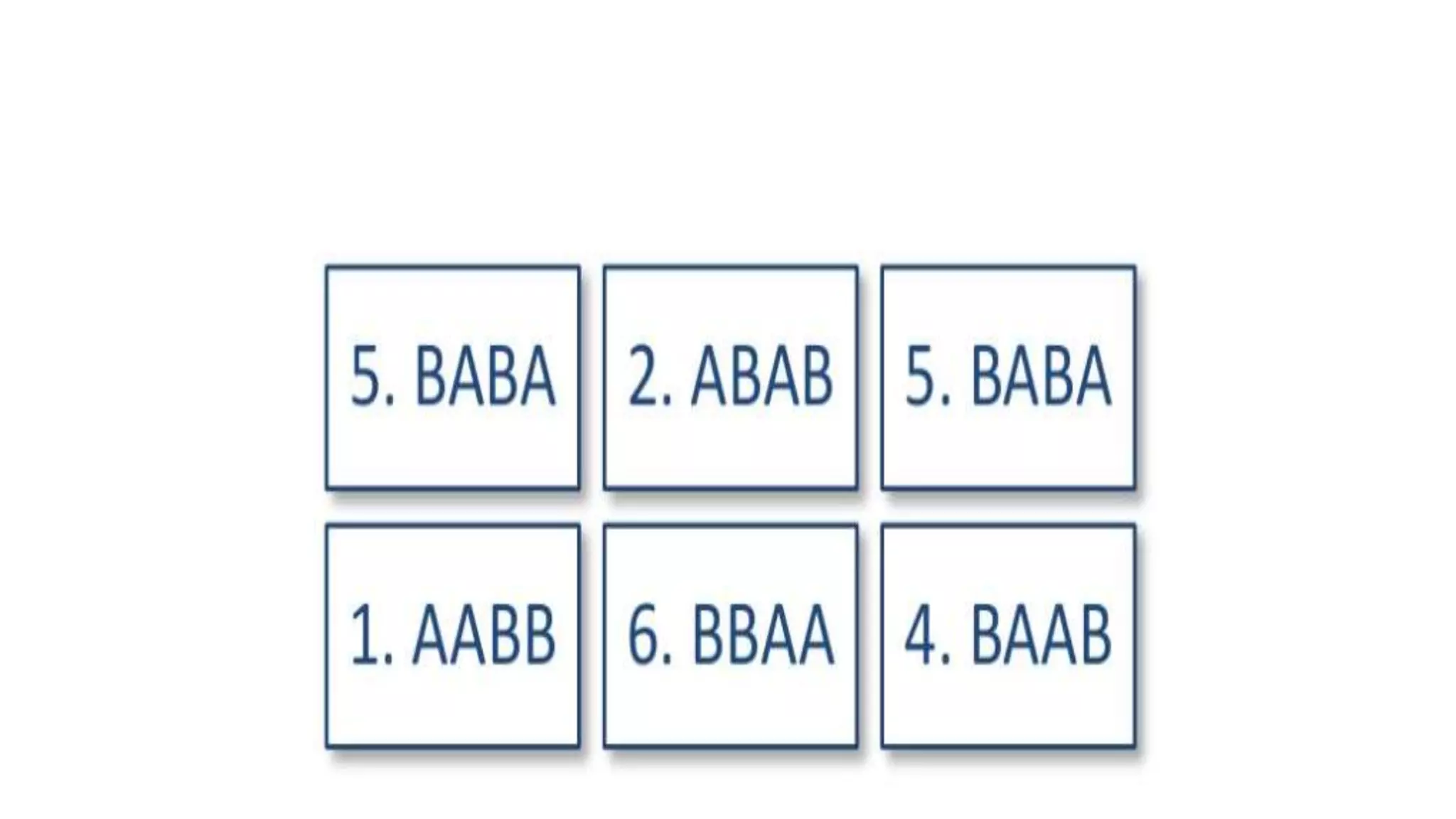
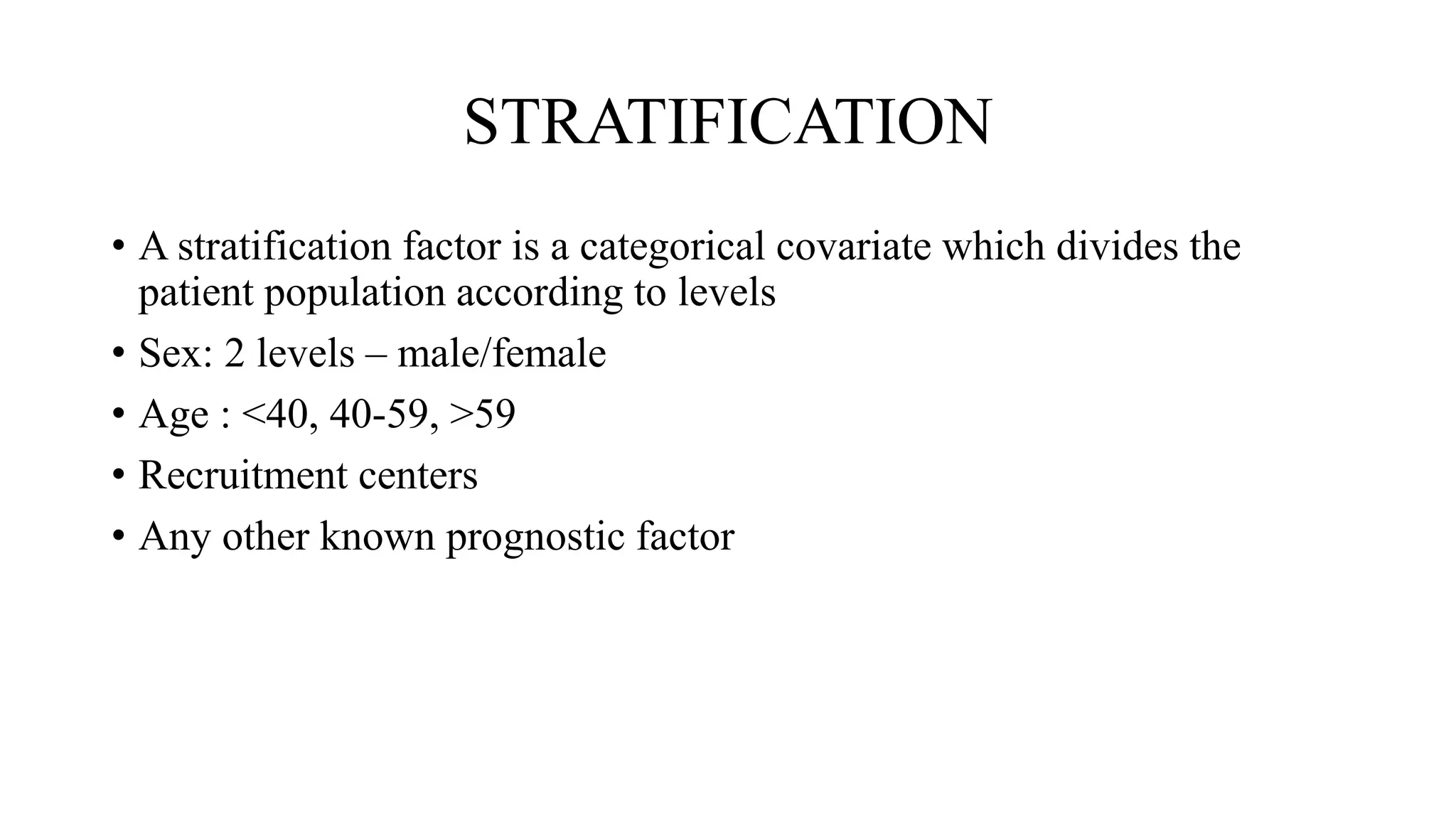
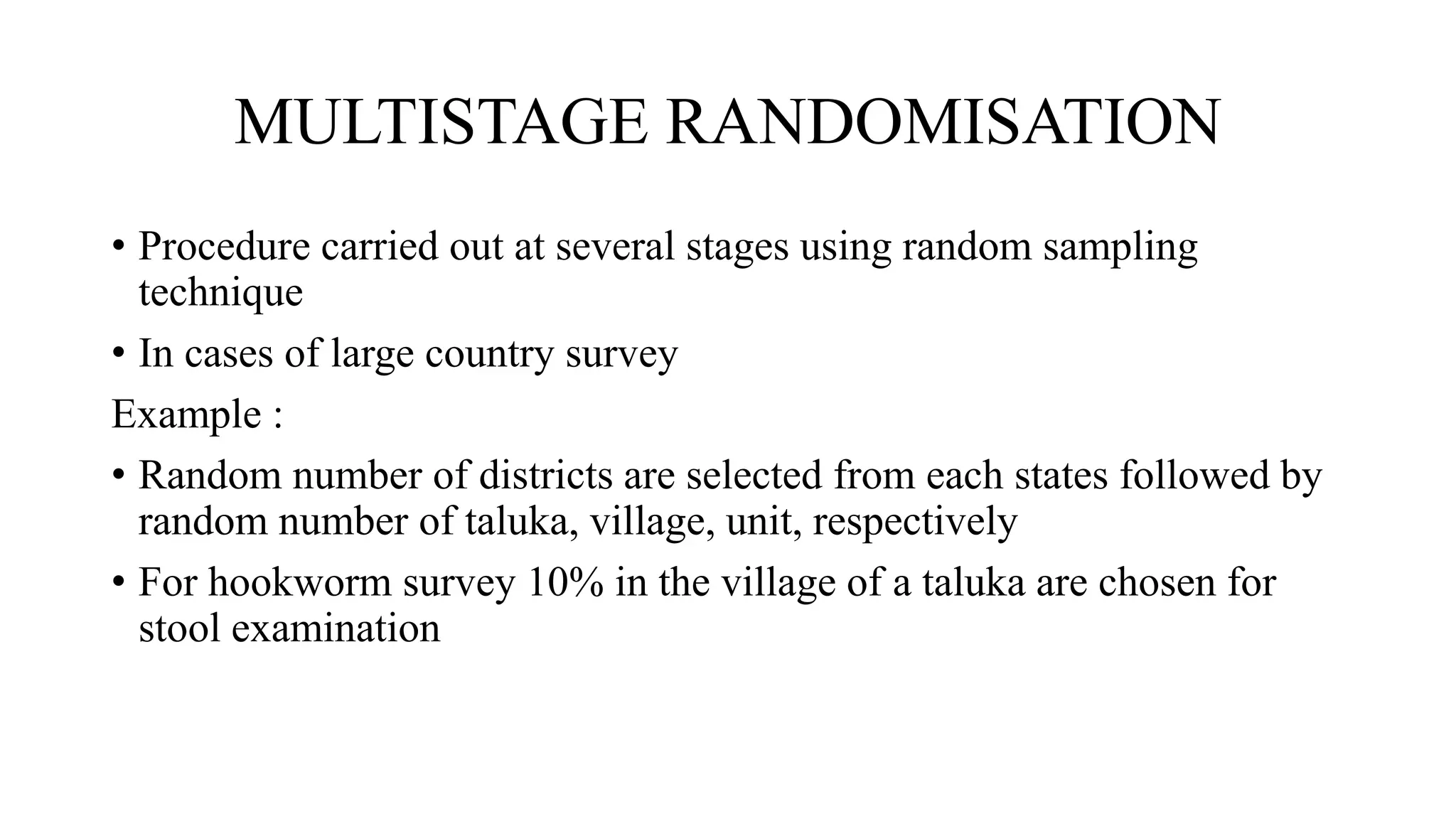
Randomisation is a process that randomly assigns participants in a clinical trial to treatment groups in order to prevent bias. It distributes characteristics of participants evenly across groups and ensures comparability. Common randomisation methods include simple randomisation using a coin flip or computer generation, block randomisation which assigns participants in blocks to balance group sizes, and stratified randomisation which divides participants with prognostic factors into subgroups before randomisation. Bias can still occur if the randomisation process is not properly implemented or if those involved in the trial are aware of participant group assignments.