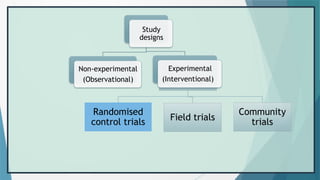
The document provides an overview of randomized controlled trials (RCTs), detailing their purpose, design, and significance in medical research. It outlines the steps involved in conducting RCTs, including protocol development, subject selection, randomization, intervention, and outcome assessment, while also discussing the advantages and disadvantages of using this research method. Additionally, it elaborates on challenges faced during RCTs, including issues of non-compliance and biases, enhancing the understanding of RCTs as essential tools in evaluating interventions.





![HISTORY
• James Lind (1716-1794)
• In 1747, on board HMS
Salisbury, he carried out one
of the first controlled clinical
trials recorded in medical
science.
• He took 12 men suffering
from similar symptoms of
scurvy, divided them into six
pairs and treated them with
remedies suggested by
previous writers.
James Lind: The man who helped to cure scurvy with lemons. BBC News [Internet]. 2016 Oct 3 [cited 2023 Jan 5];
Available from: https://www.bbc.com/news/uk-england-37320399](https://image.slidesharecdn.com/randomizedcontroltrials-241209173029-93bd6aea/85/RANDOMIZED-CONTROL-TRIALS-PowerPoint-presentation-8-320.jpg)



![SCHEMATIC DIAGRAM
Houle S. An Introduction to the Fundamentals of Randomized Controlled Trials in Pharmacy Research. Can J Hosp Pharm
[Internet]. 2015 Feb 26](https://image.slidesharecdn.com/randomizedcontroltrials-241209173029-93bd6aea/85/RANDOMIZED-CONTROL-TRIALS-PowerPoint-presentation-13-320.jpg)

















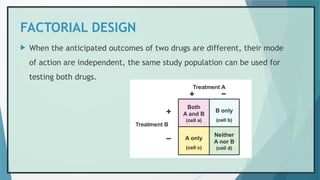


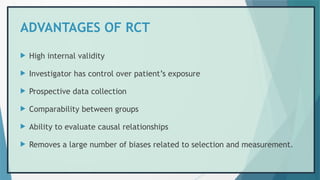
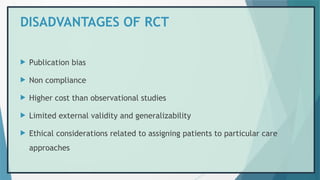












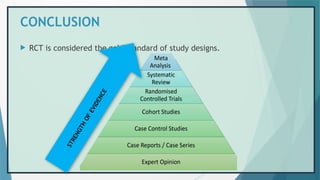

![REFERENCES
1. Bonita R, Beaglehole R, Kjellström T. Basic epidemiology. 2nd ed. Geneva: World health
Organisation; 2014. 212 p.
2. James Lind: The man who helped to cure scurvy with lemons. BBC News [Internet]. 2016 Oct 3
[cited 2023 Jan 5]; Available from: https://www.bbc.com/news/uk-england-37320399
3. Celentano DD, Szklo M. Gordis epidemiology. 6th ed. New Delhi: RELIX india pvt ltd, Elsevier;
2020. 420 p.
4. Houle S. An Introduction to the Fundamentals of Randomized Controlled Trials in Pharmacy
Research. Can J Hosp Pharm [Internet]. 2015 Feb 26 [cited 2023 Jan 6];68(1). Available from:
https://www.cjhp-online.ca/index.php/cjhp/article/view/1422
5. Park K. Park’s textbook pf preventive and social medicine. 26th ed. Jabalpur: M/s Banarsidas
Bhanot publishers; 2021.](https://image.slidesharecdn.com/randomizedcontroltrials-241209173029-93bd6aea/85/RANDOMIZED-CONTROL-TRIALS-PowerPoint-presentation-55-320.jpg)
![REFERENCES
6. Hall NS. R. A. Fisher and his advocacy of randomization. J Hist Biol. 2007;40(2):295–325.
7. Bhide A, Shah PS, Acharya G. A simplified guide to randomized controlled trials. Acta Obstet
Gynecol Scand. 2018;97(4):380–7.
8. Saghaei M. An Overview of Randomization and Minimization Programs for Randomized Clinical
Trials. J Med Signals Sens. 2011;1(1):55–61.
9. Appendix 7.A Using a Table of Random Numbers - Statistical Inference: A Short Course [Book]
[Internet]. [cited 2023 Jan 8]. Available from: https://www.oreilly.com/library/view/statistical-
inference-a/9781118309803/c07anchor-6.html
10. Katz D, Elmore J, wild D, Lucan S. Jekel’s Epidemiology, Biostatistics, Preventive Medicine, and
Public Health. 4th ed. Philadelphiaa: Elsevier saunders; 2014.](https://image.slidesharecdn.com/randomizedcontroltrials-241209173029-93bd6aea/85/RANDOMIZED-CONTROL-TRIALS-PowerPoint-presentation-56-320.jpg)
![REFERENCES
11. Bhalwar R. Textbook of public health and community medicine. 3rd ed. New Delhi: Wolters
Kluwer Pvt Ltd; 2019.
12. Clinical Trials Registry - India (CTRI) [Internet]. [cited 2023 Jan 8]. Available from:
https://ctri.nic.in/Clinicaltrials/login.php
13. QUASI- | English meaning - Cambridge Dictionary [Internet]. [cited 2023 Jan 9]. Available from:
https://dictionary.cambridge.org/dictionary/english/quasi
14. Medical Respiratory Intensive Care Unit Nursing, Fowler AA, Syed AA, Knowlson S, Sculthorpe R,
Farthing D, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J
Transl Med. 2014 Dec;12(1):32.
15. Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, et al. Effect of Vitamin C,
Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor
Support Among Patients With Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA. 2020
Feb 4;323(5):423–31.](https://image.slidesharecdn.com/randomizedcontroltrials-241209173029-93bd6aea/85/RANDOMIZED-CONTROL-TRIALS-PowerPoint-presentation-57-320.jpg)
