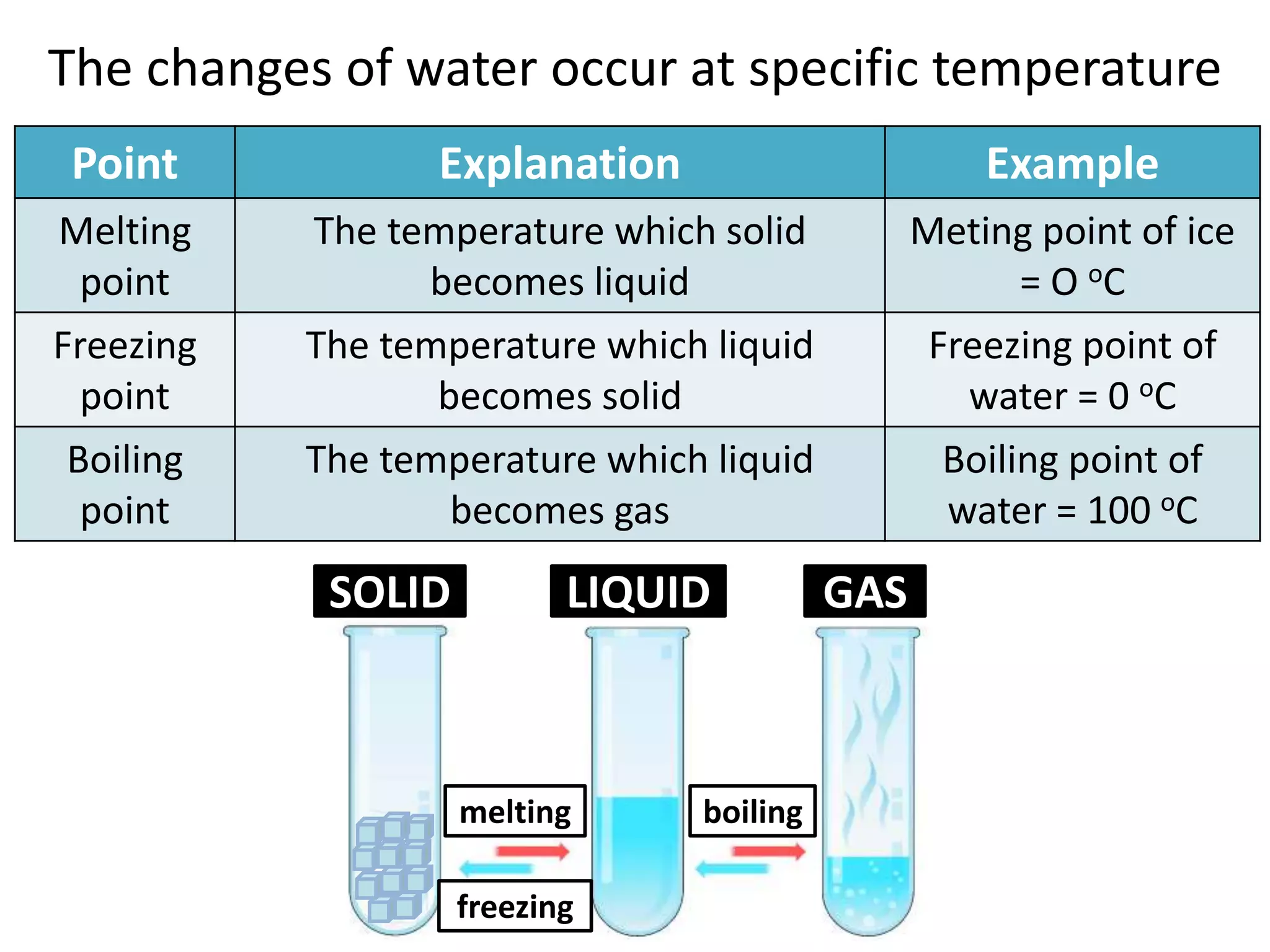
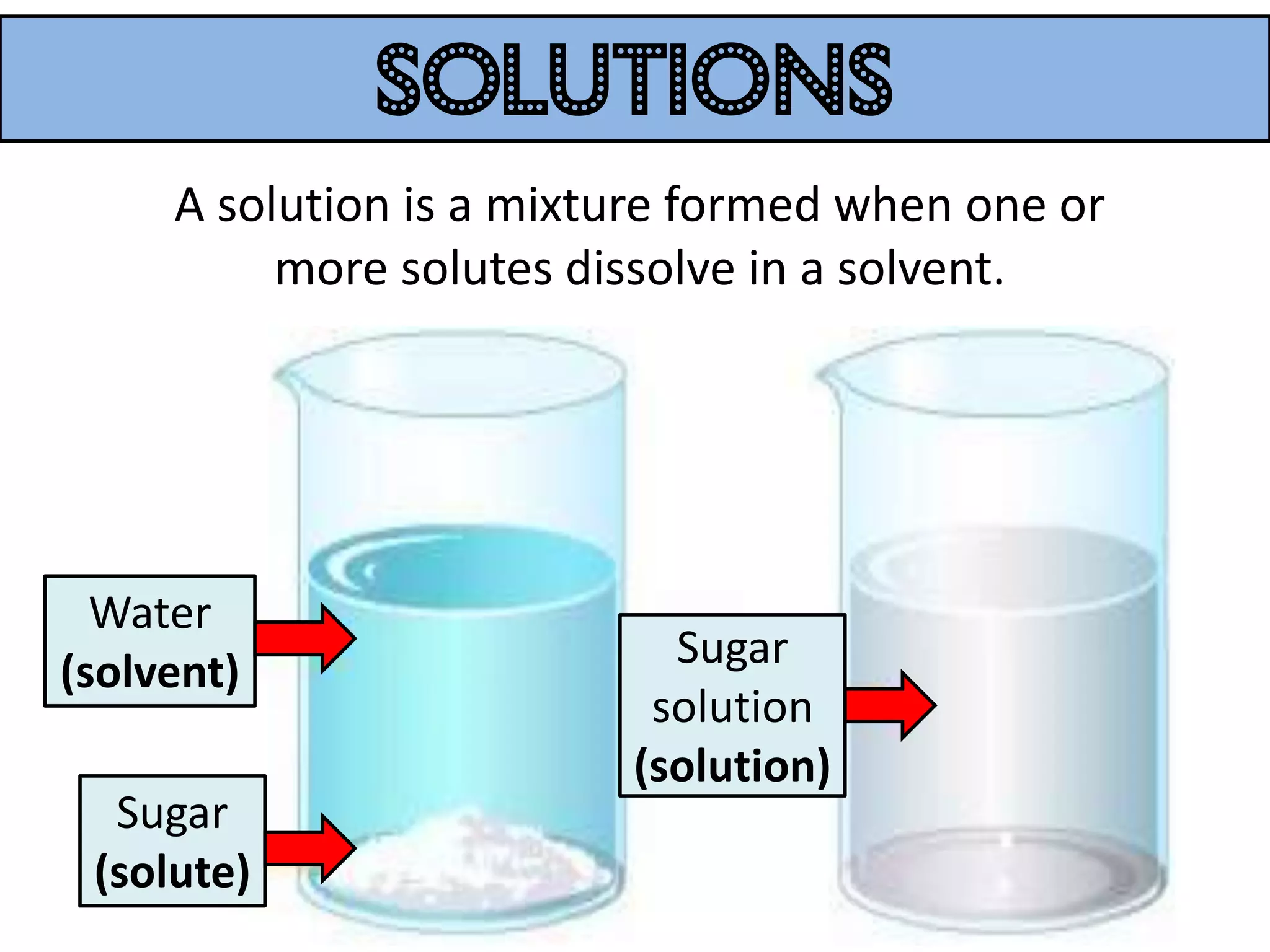
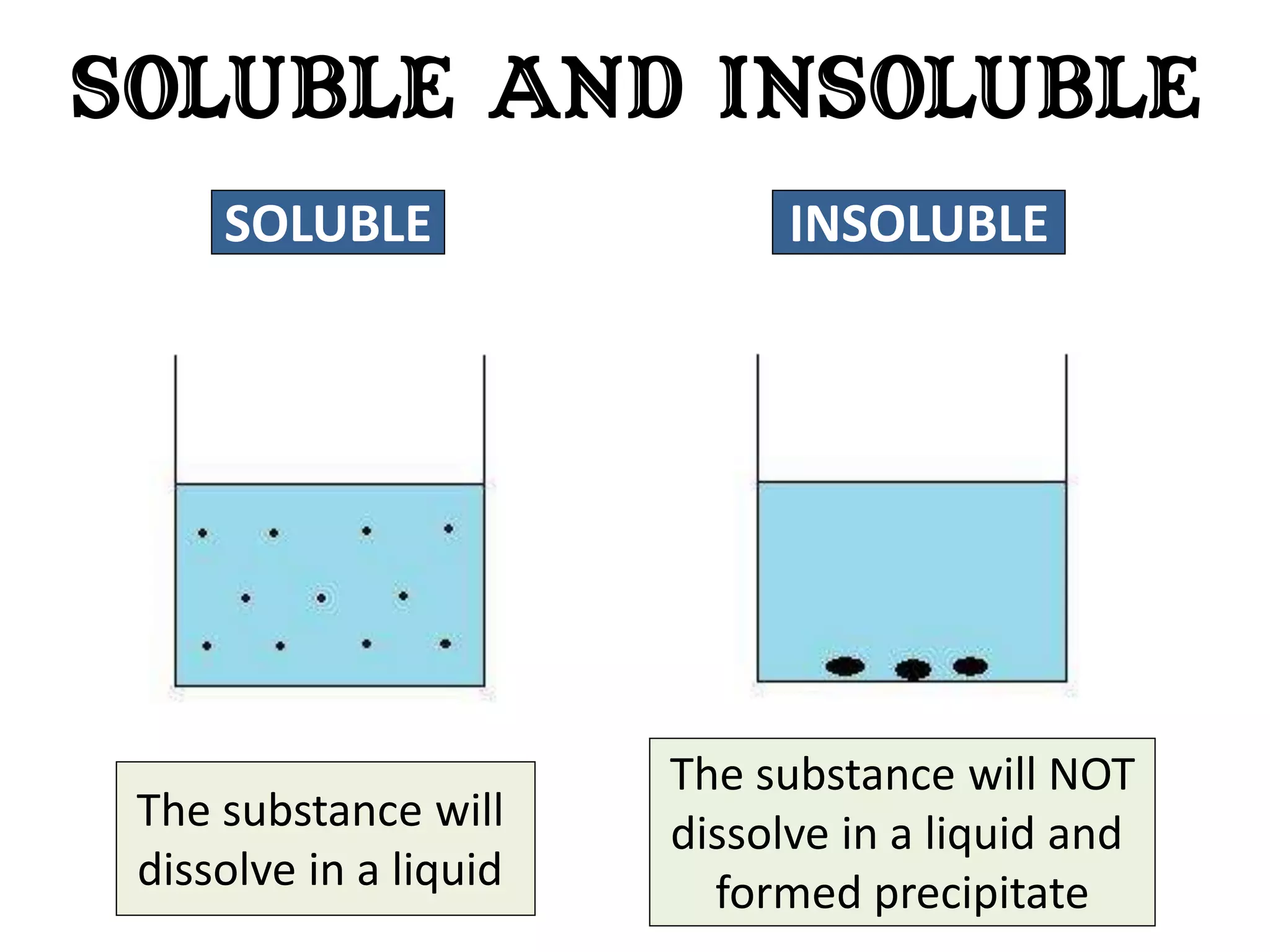
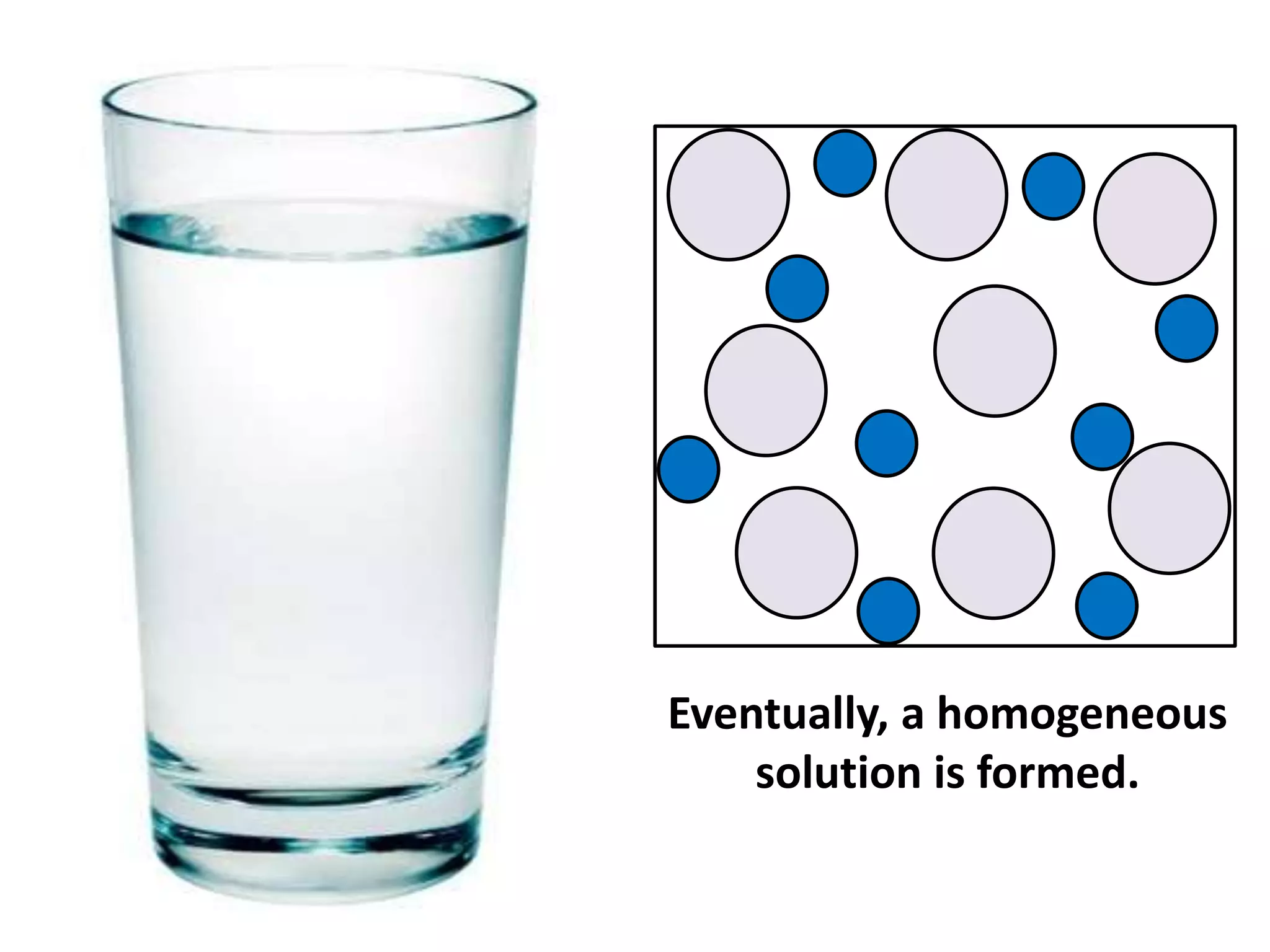
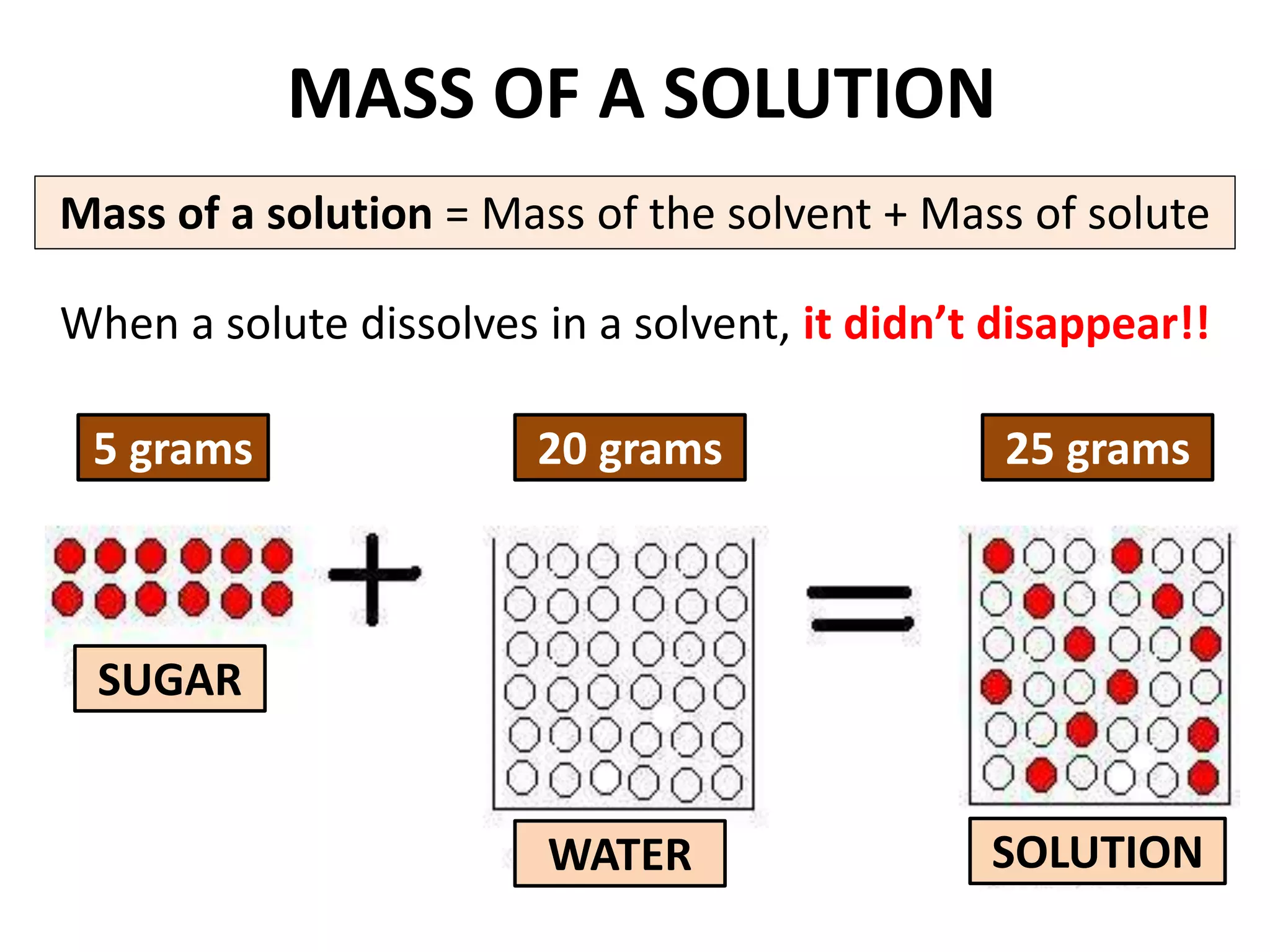
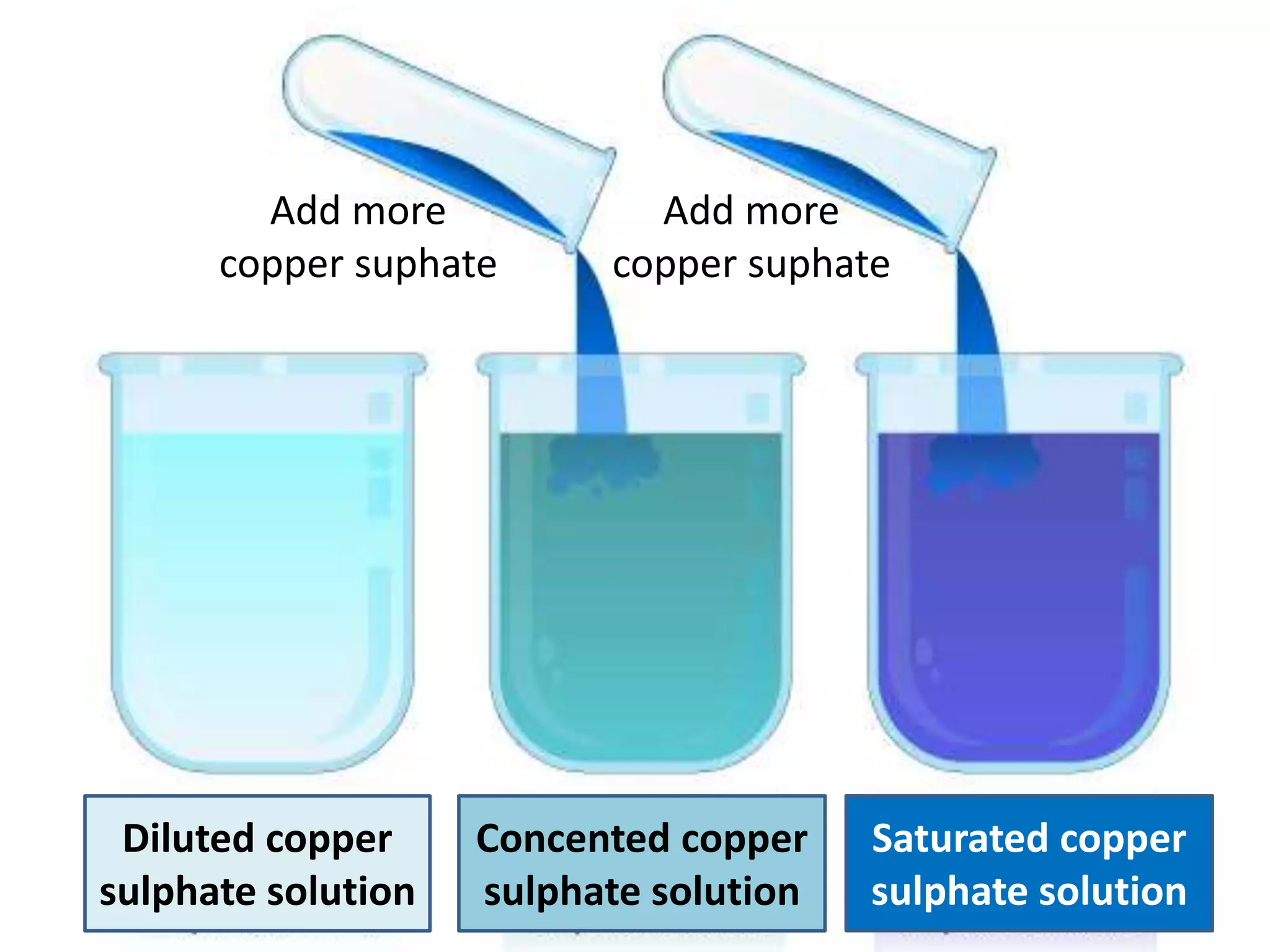
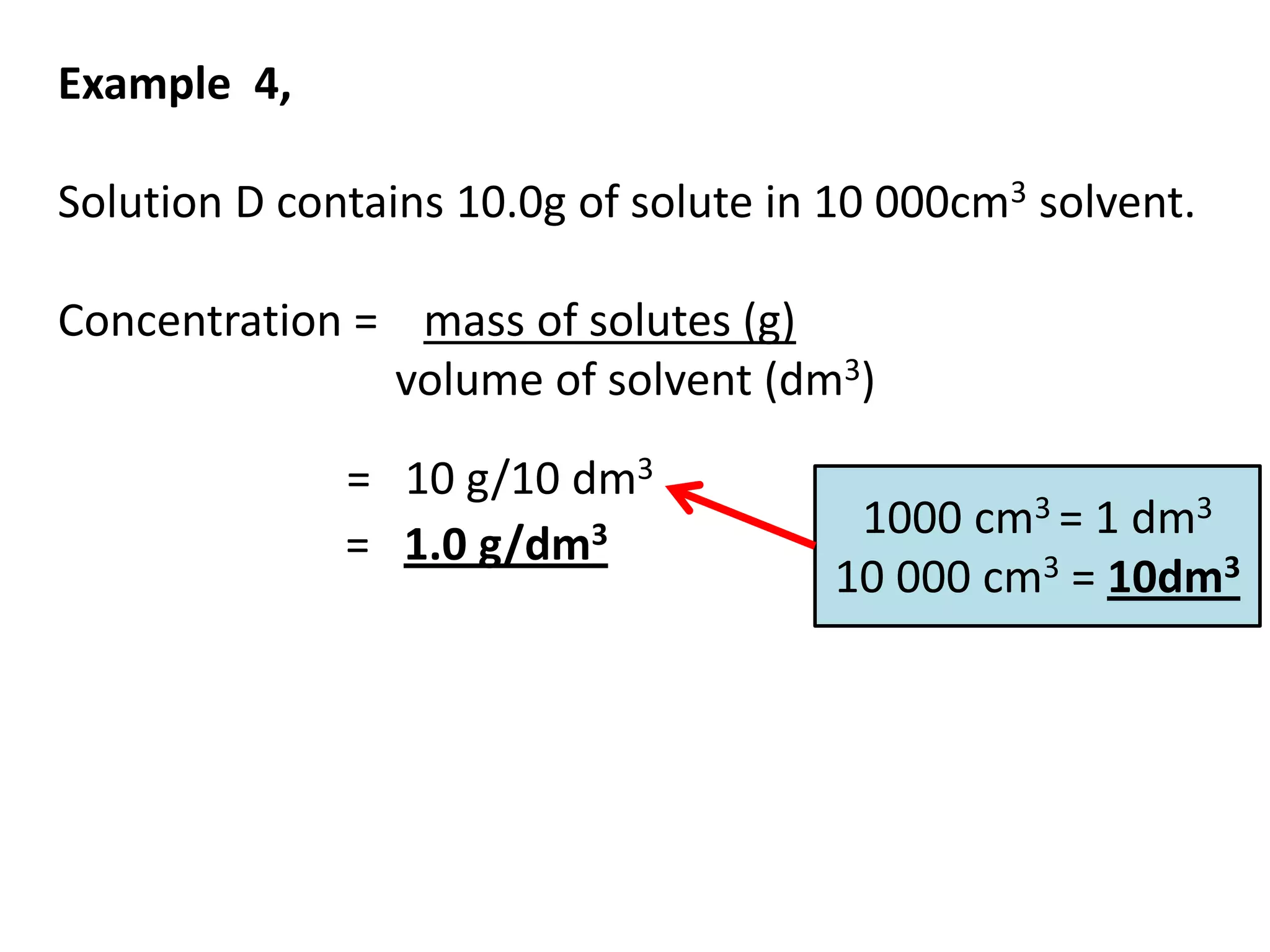
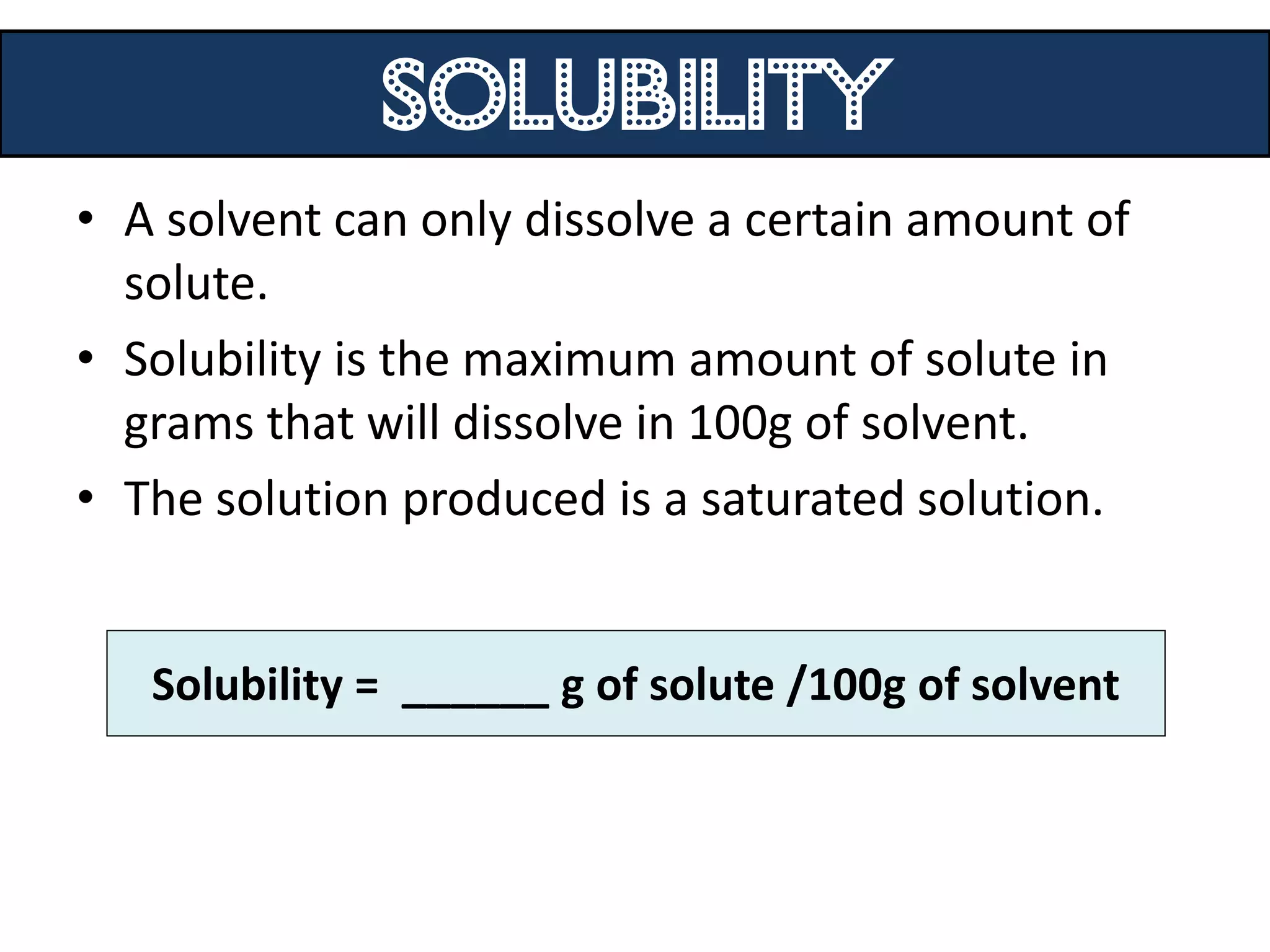
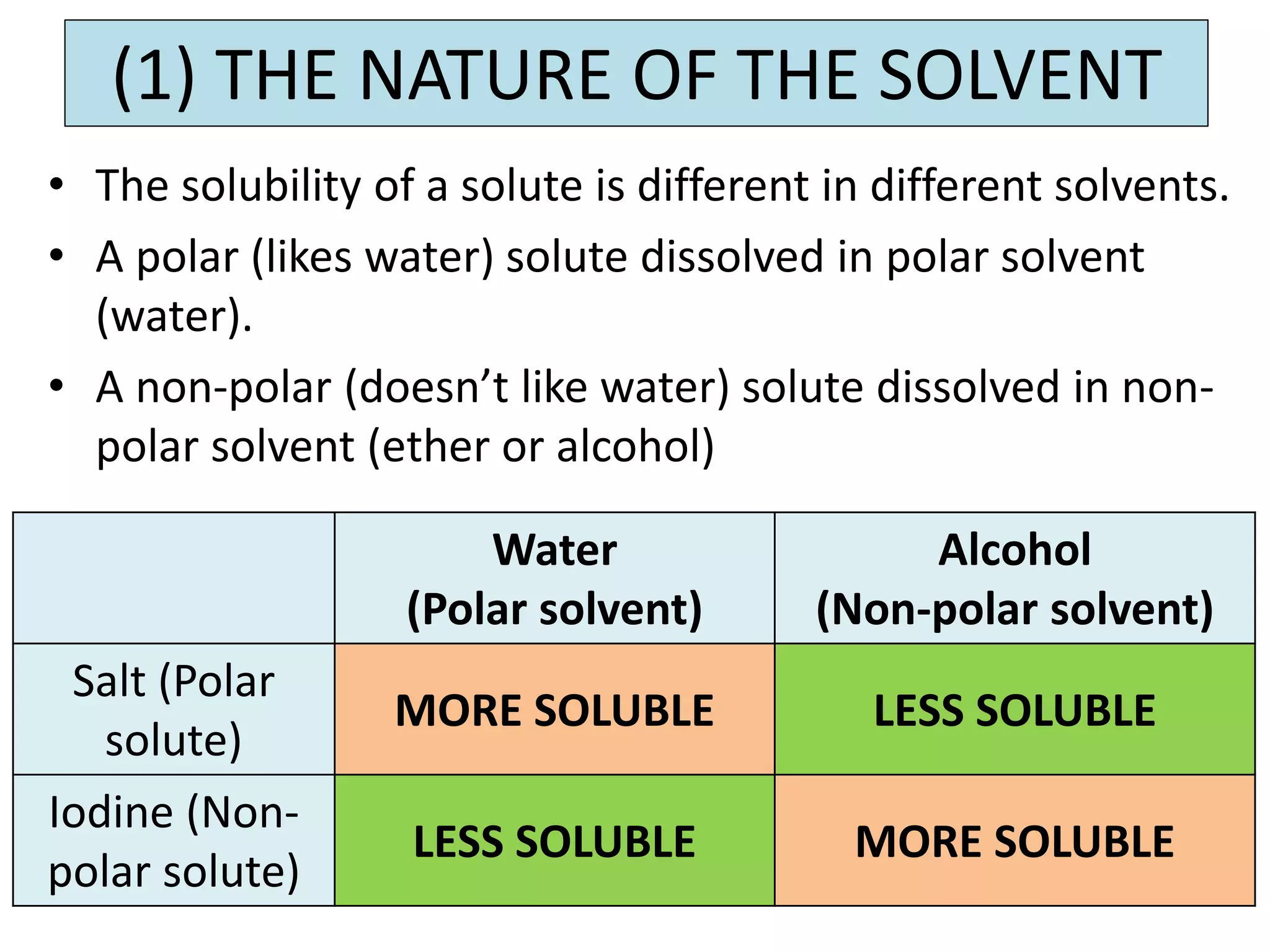
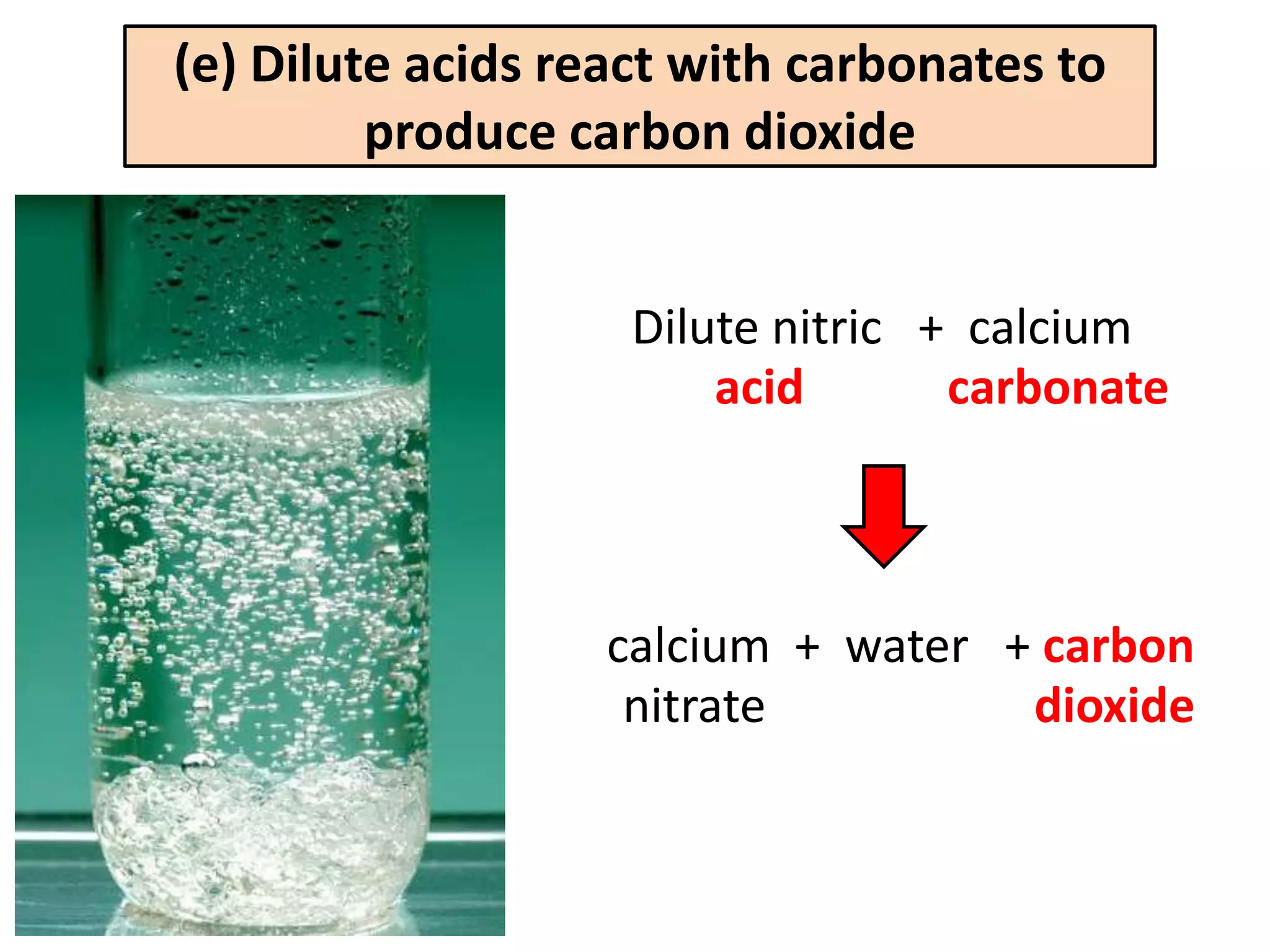
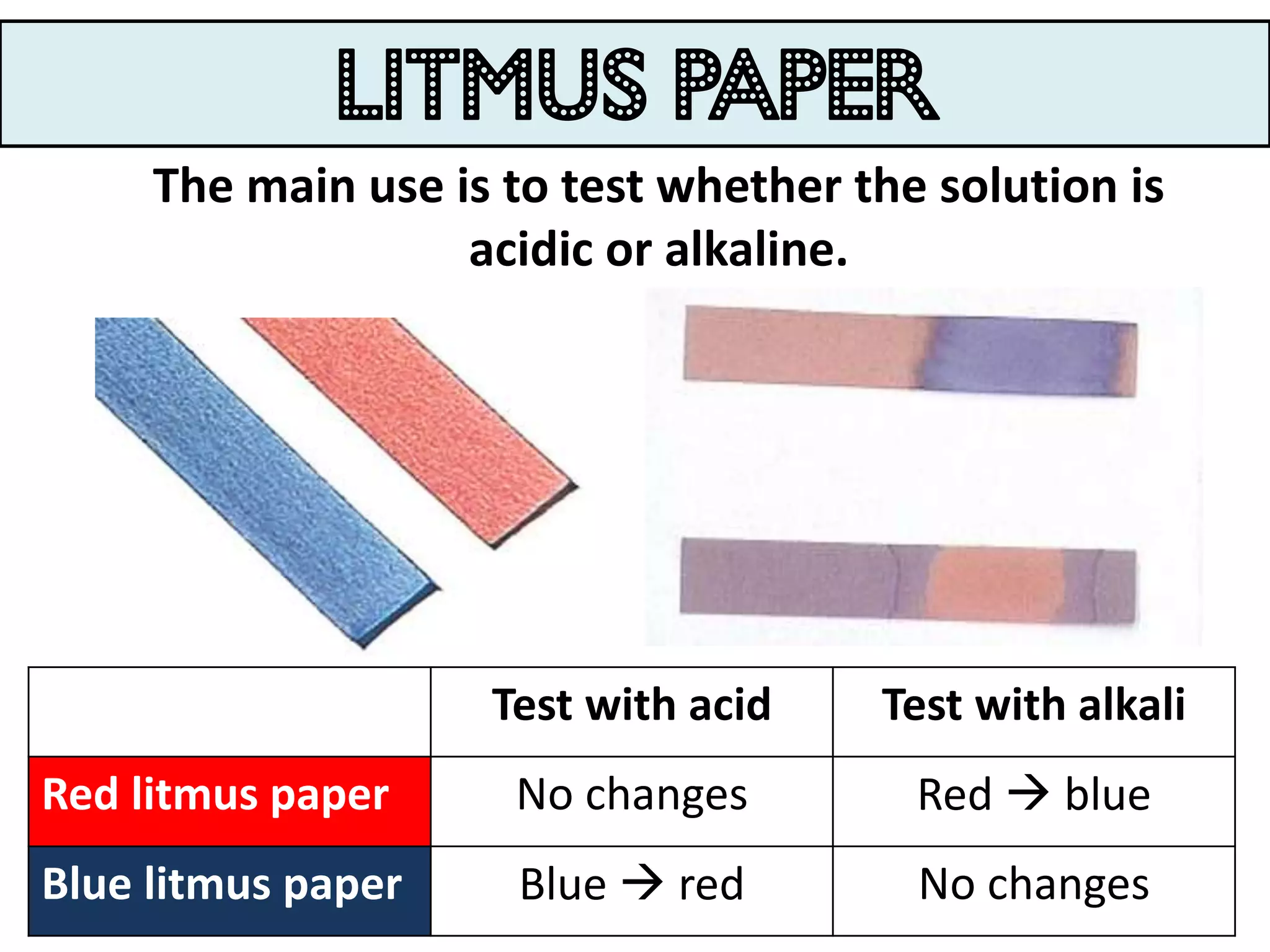
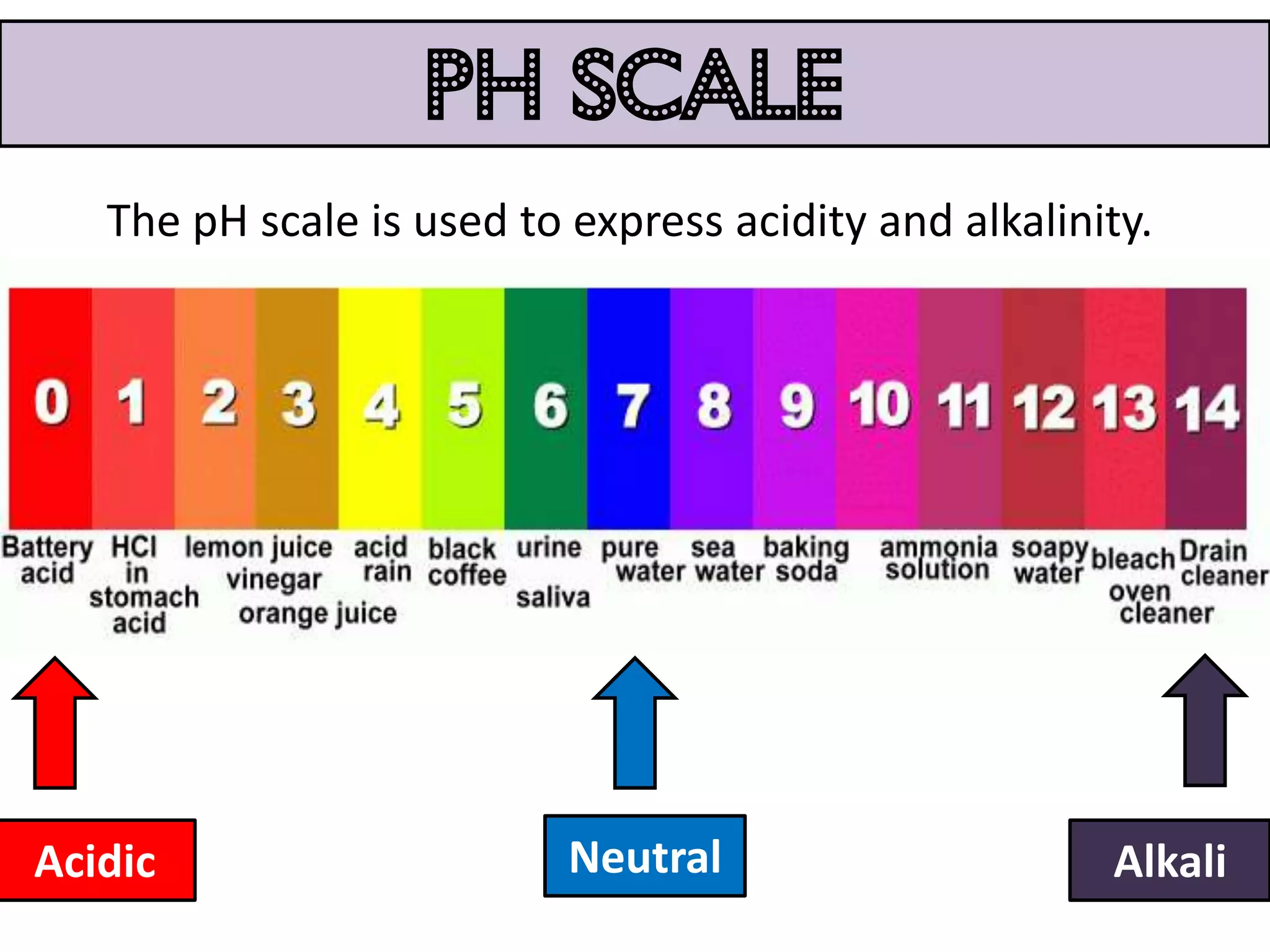
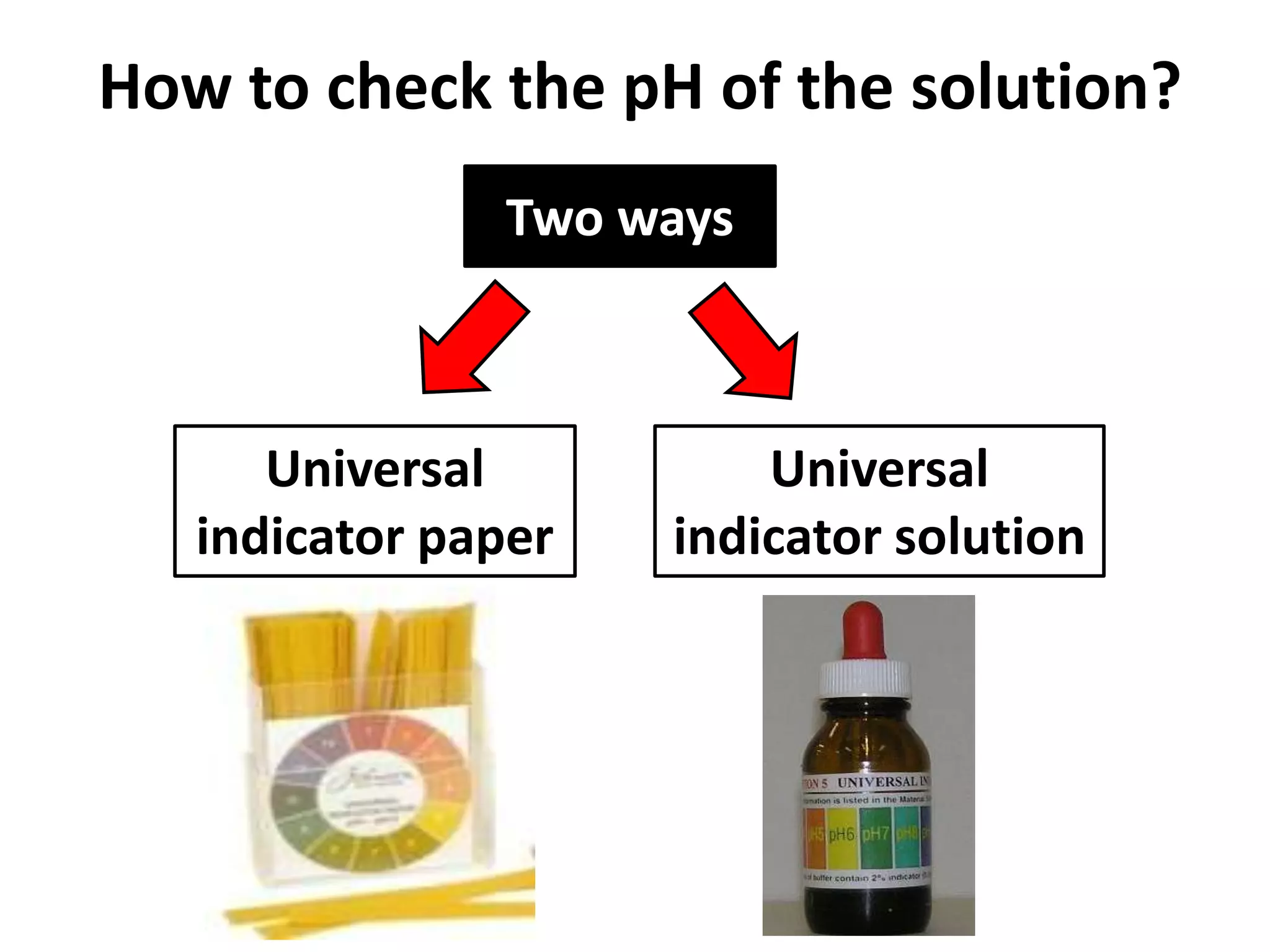
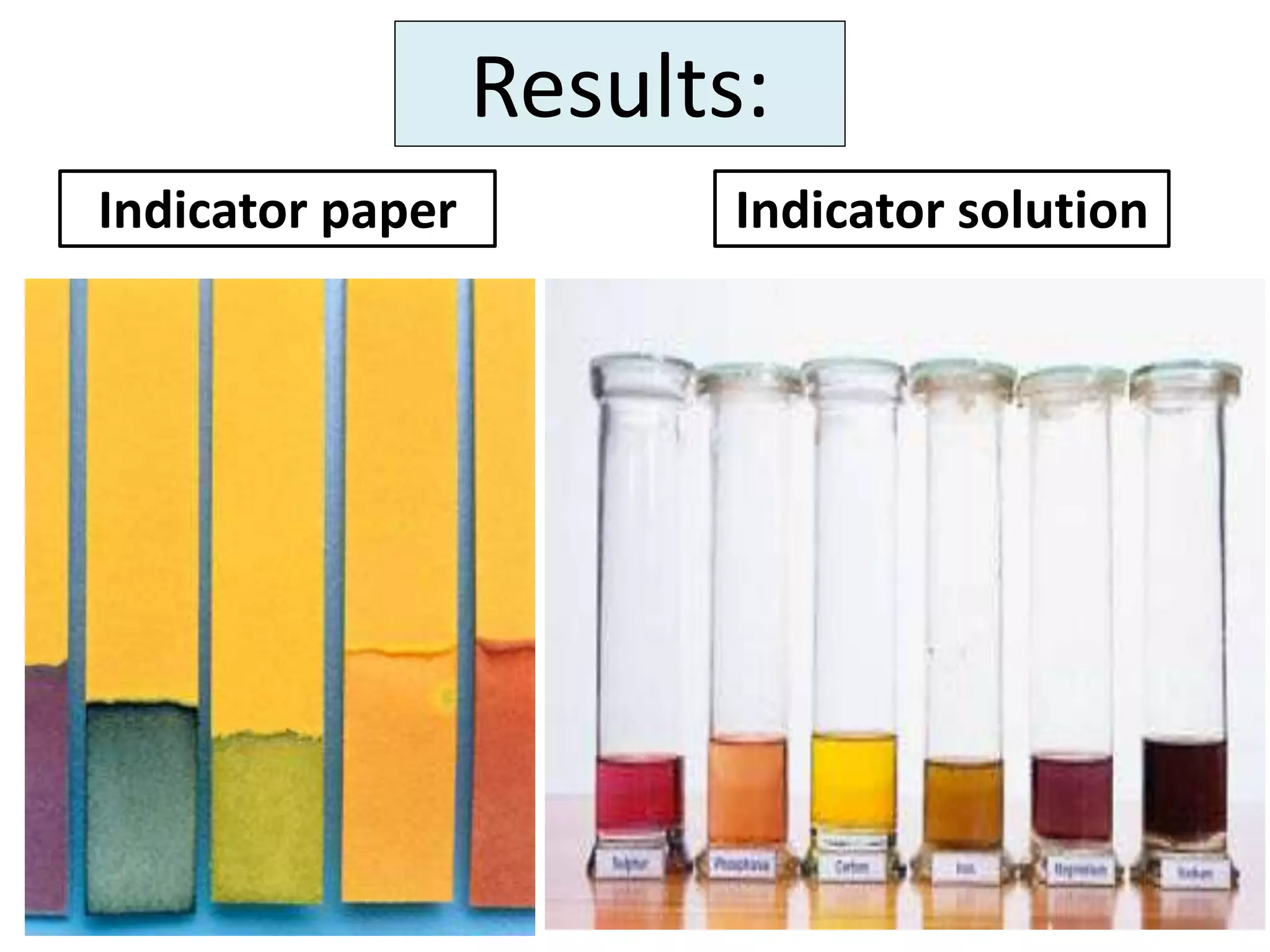
Water can exist in three states - solid, liquid, and gas - and the changes between these states occur at specific temperatures. A solution is a homogeneous mixture formed when one or more solutes dissolve in a solvent like water. The solubility of a substance is affected by factors such as the nature of the solute and solvent as well as temperature, with solubility generally increasing as temperature rises.