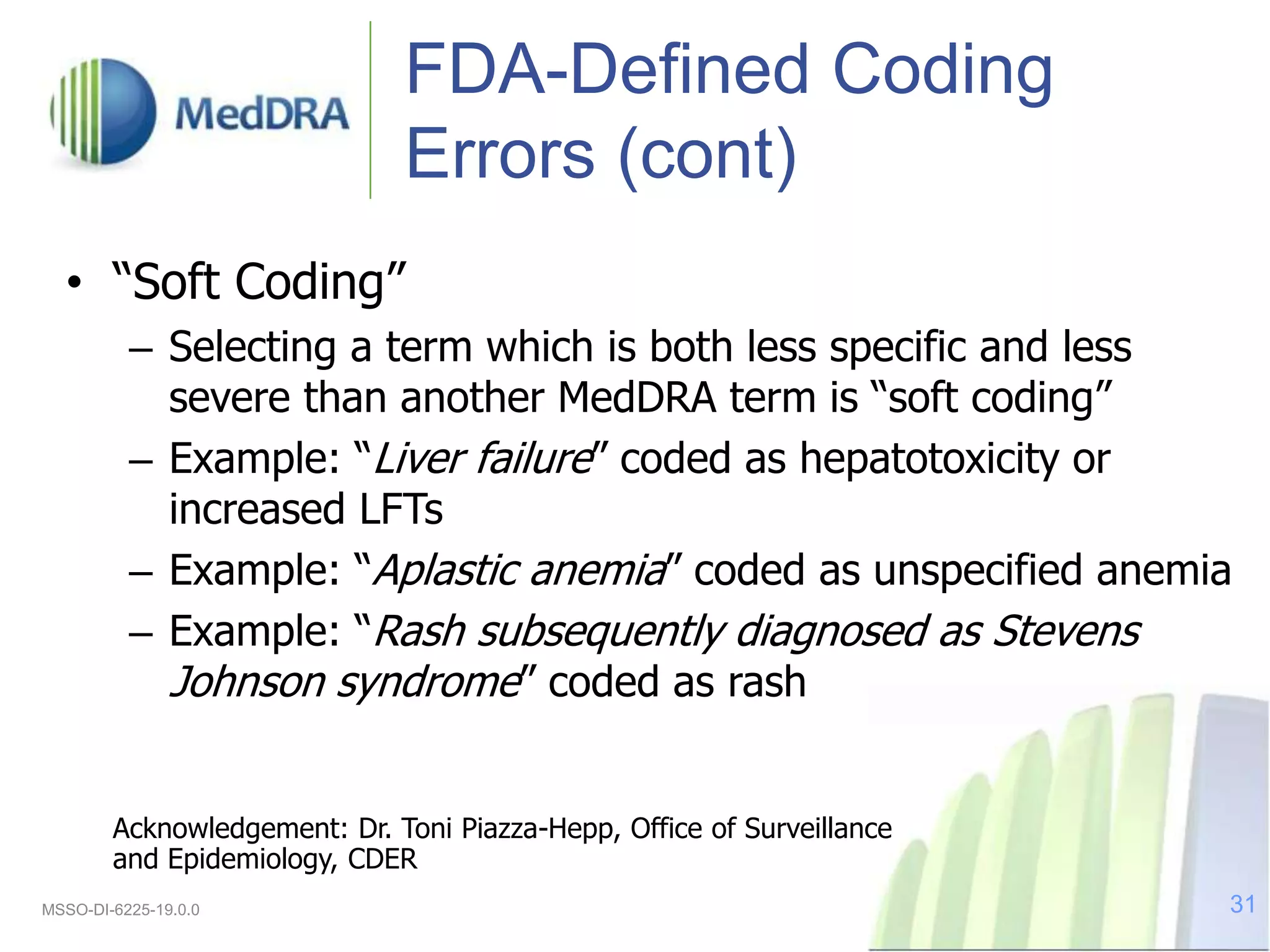
The document discusses the importance of data quality in clinical development, emphasizing the role of MEDDRA, an internationally recognized medical terminology for regulatory activities. It outlines how data is coded, the significance of accurate reporting, and the challenges faced in achieving quality data due to ambiguities and coding errors. Ultimately, the presentation stresses the benefits of good quality data for improving communication and safety in clinical trials and regulatory processes.