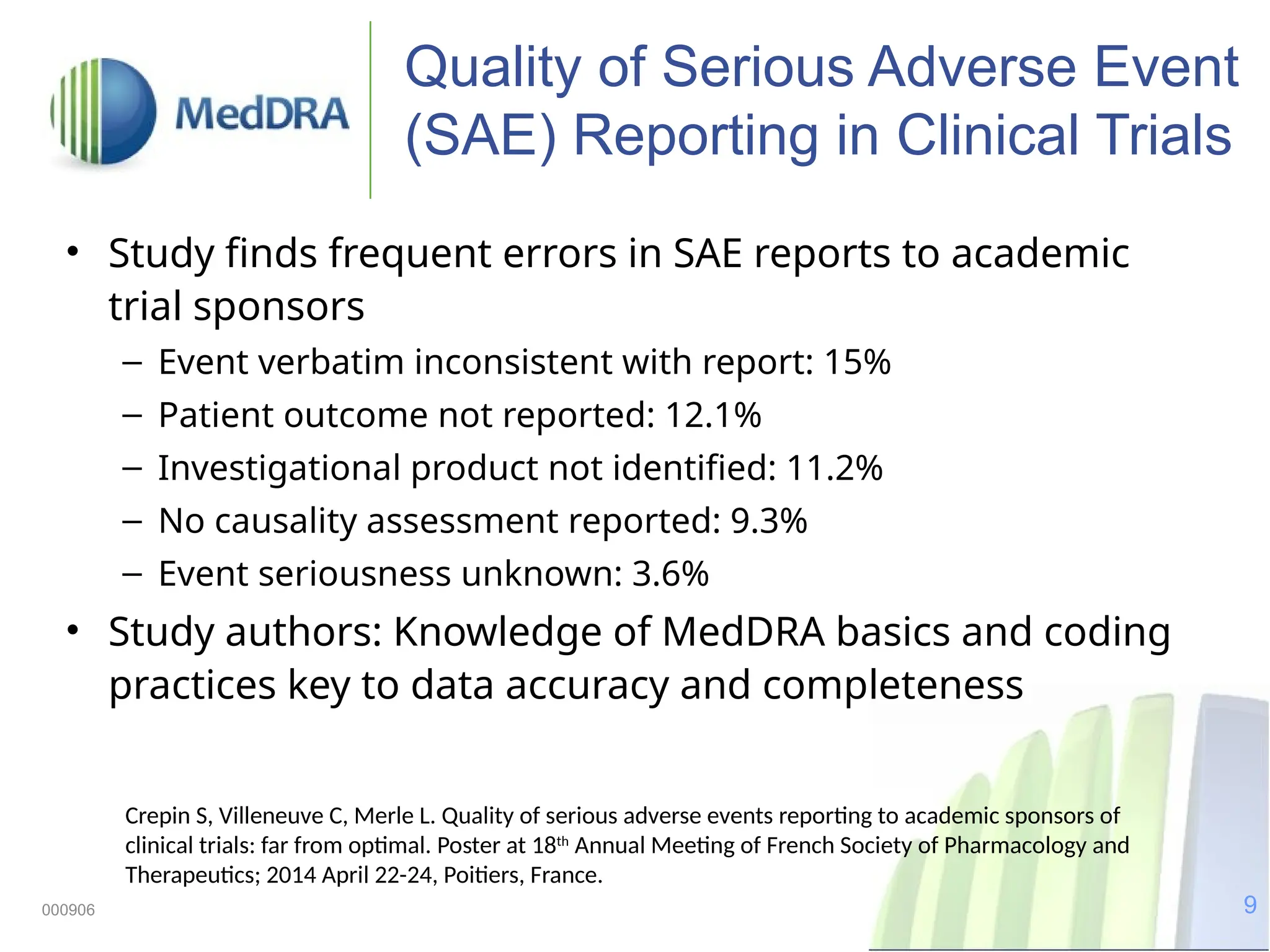
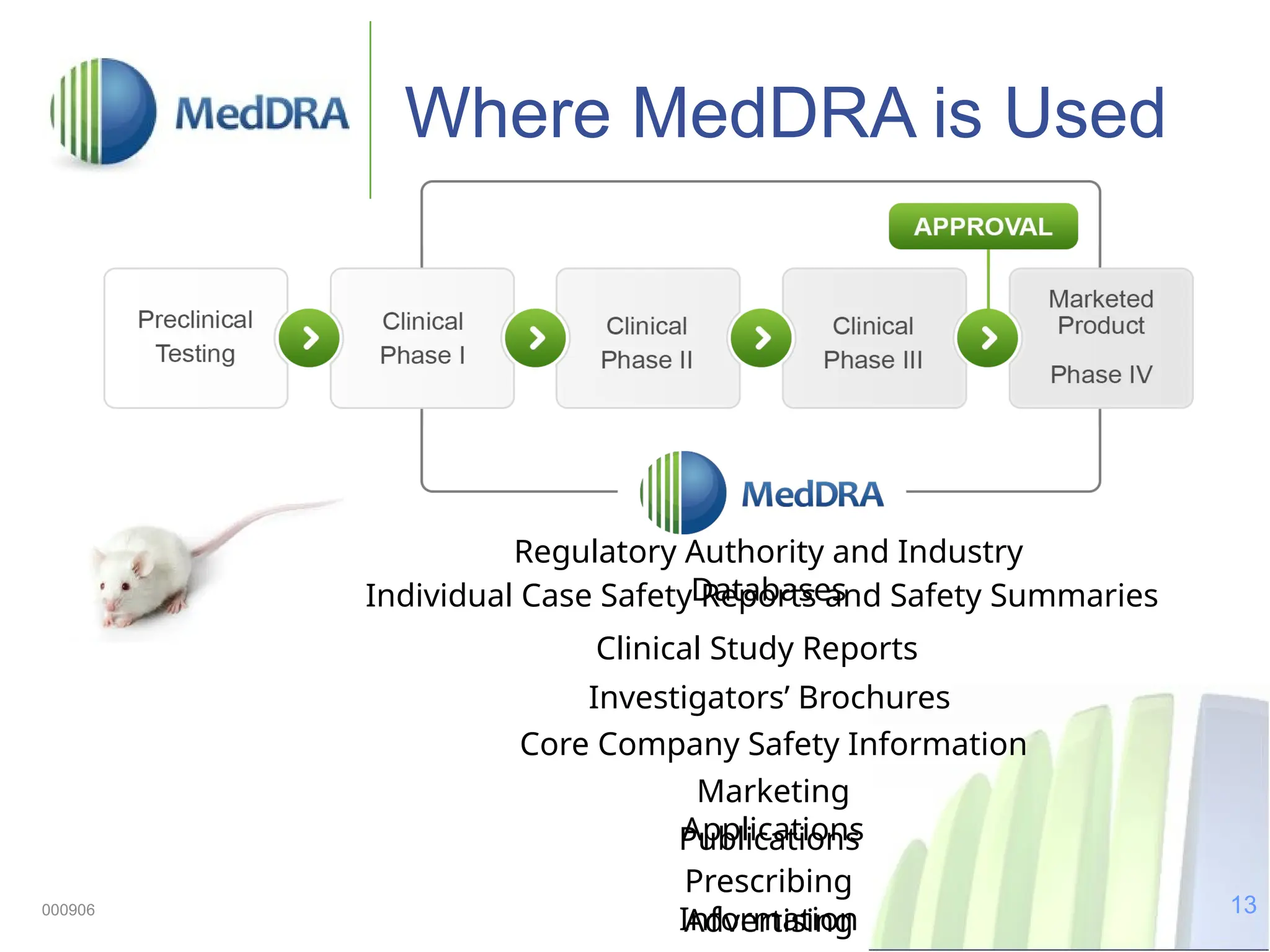
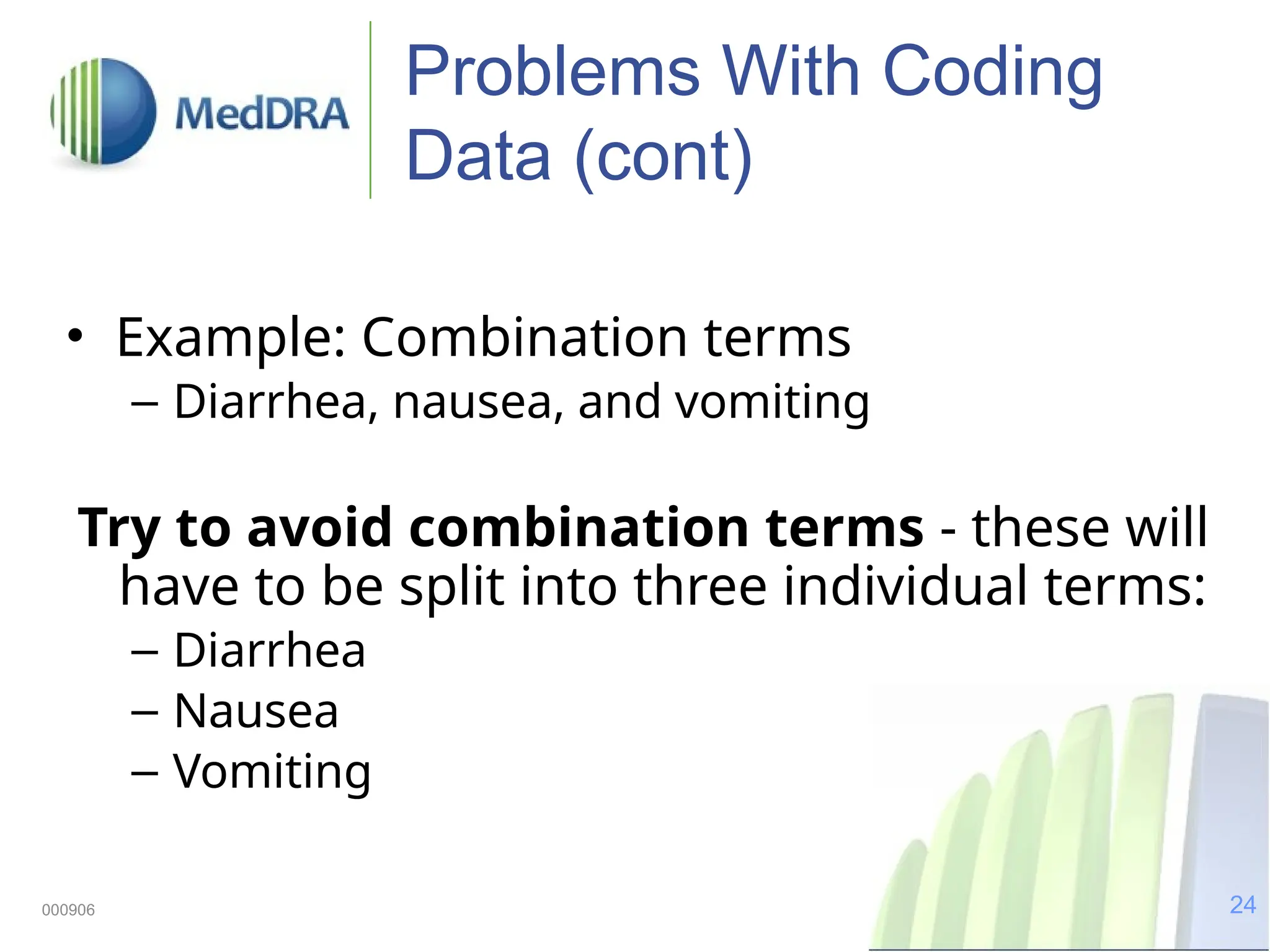
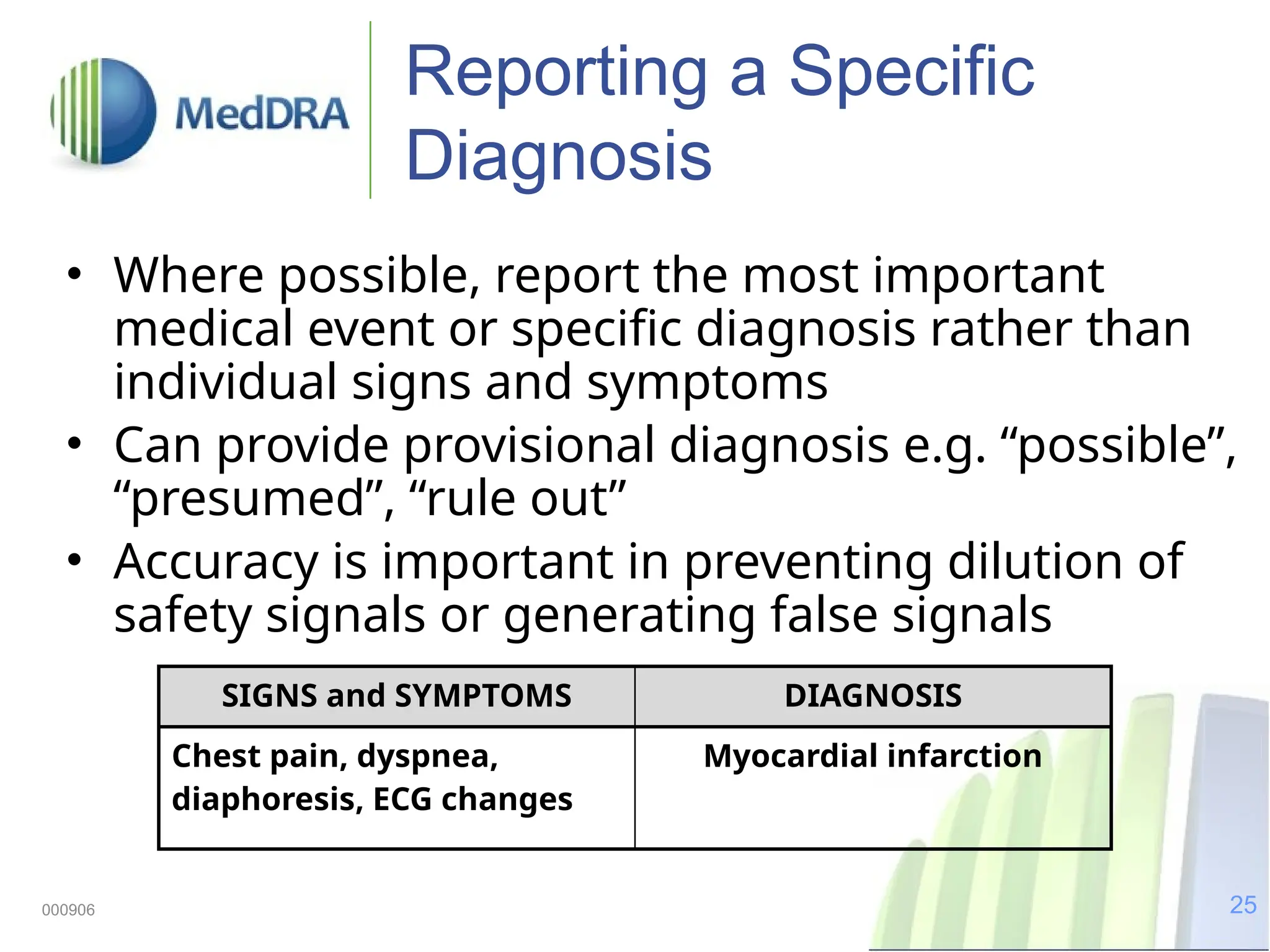
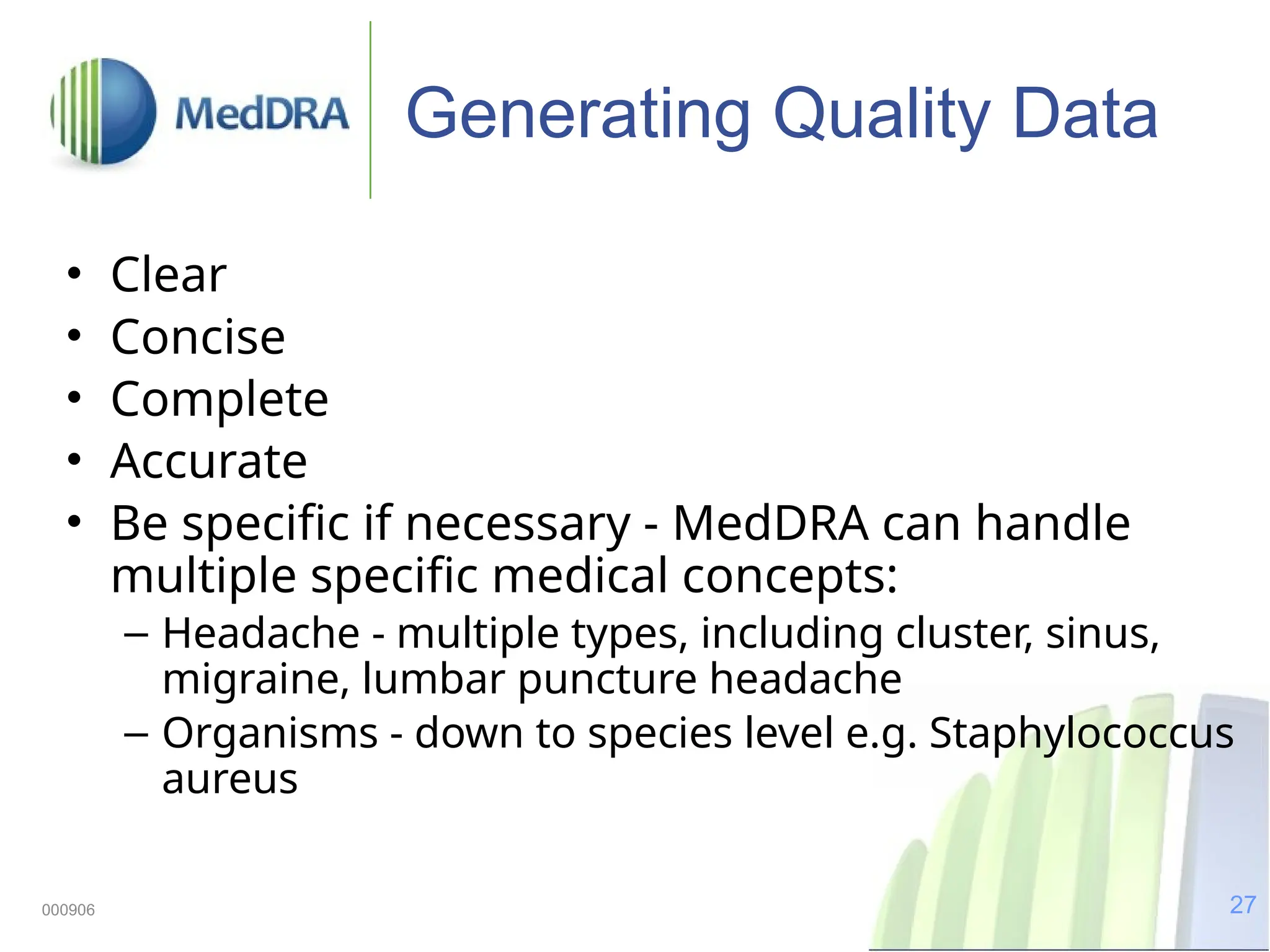
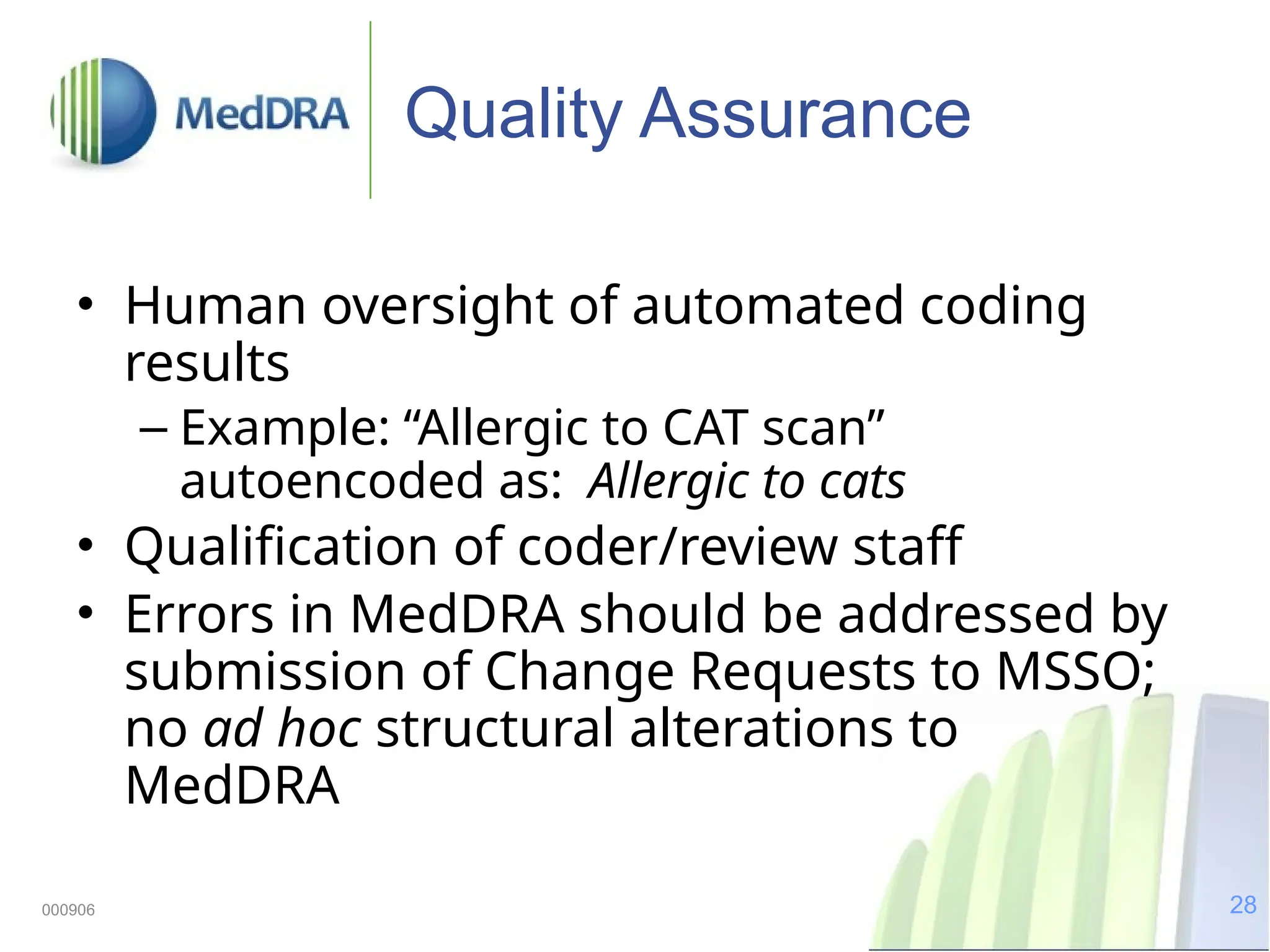
The document discusses the importance of high-quality data in clinical trials and the role of the Medical Dictionary for Regulatory Activities (MedDRA) in ensuring accurate coding and reporting of clinical data. It highlights the significance of clear, complete, and precise data entry to prevent coding errors and enhance safety signal detection in drug safety surveillance. Additionally, it provides insights into MedDRA's structure, features, and operational procedures for maintaining data quality.