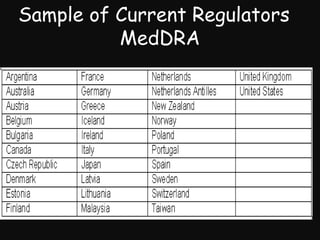
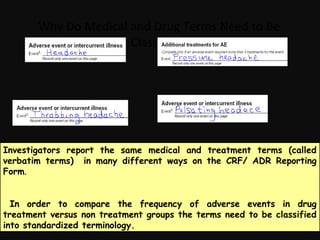
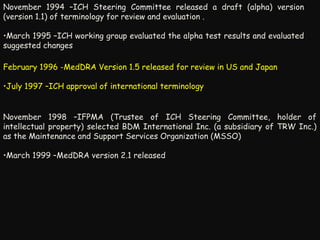
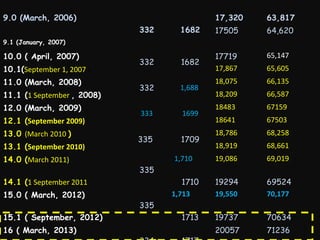
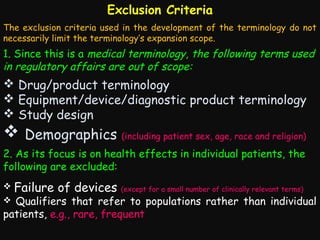
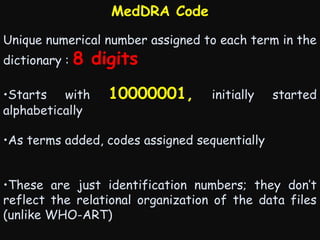
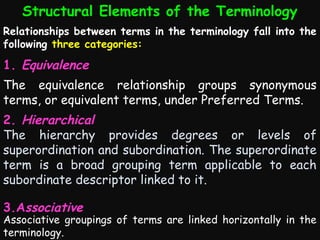
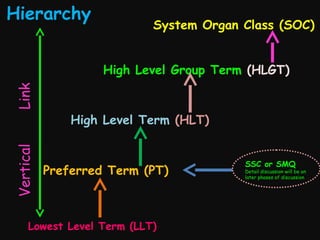
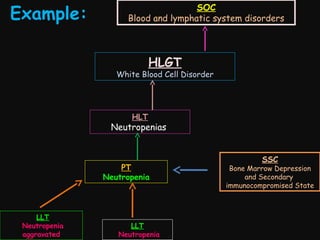
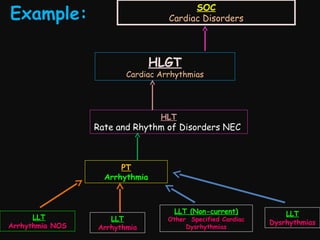
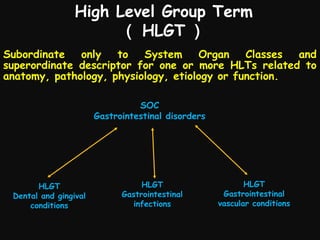
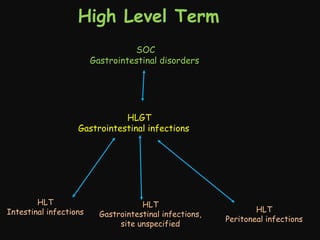
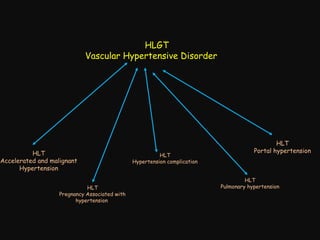
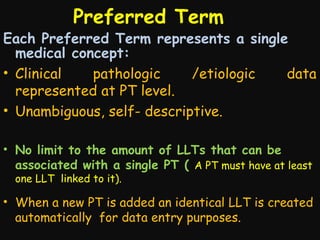
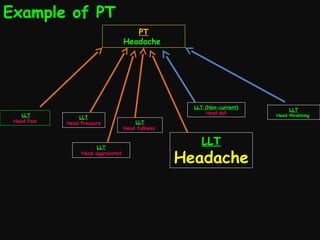
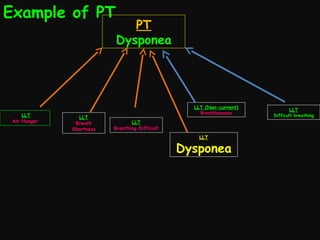
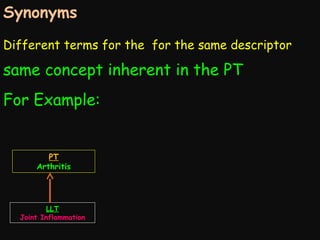
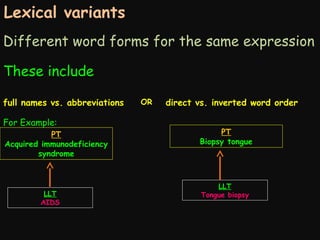
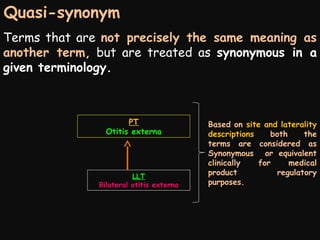
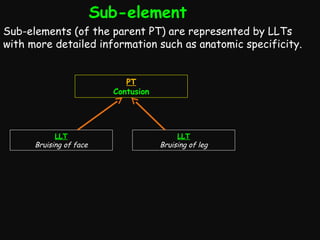
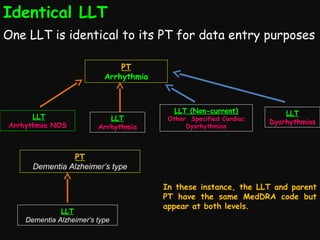
This document provides an introduction and overview of MedDRA, the medical terminology used for medical coding in the pharmaceutical industry. It discusses the history and development of MedDRA, which originated from the need for a single, standardized terminology to classify medical information for regulatory purposes. The document outlines the requirements for MedDRA, how it addresses issues with prior terminologies, and its scope and uses in clinical research and by regulatory authorities. It also provides examples of the structural elements of MedDRA and how it has been expanded over multiple versions to include more terms and enhance functionality.