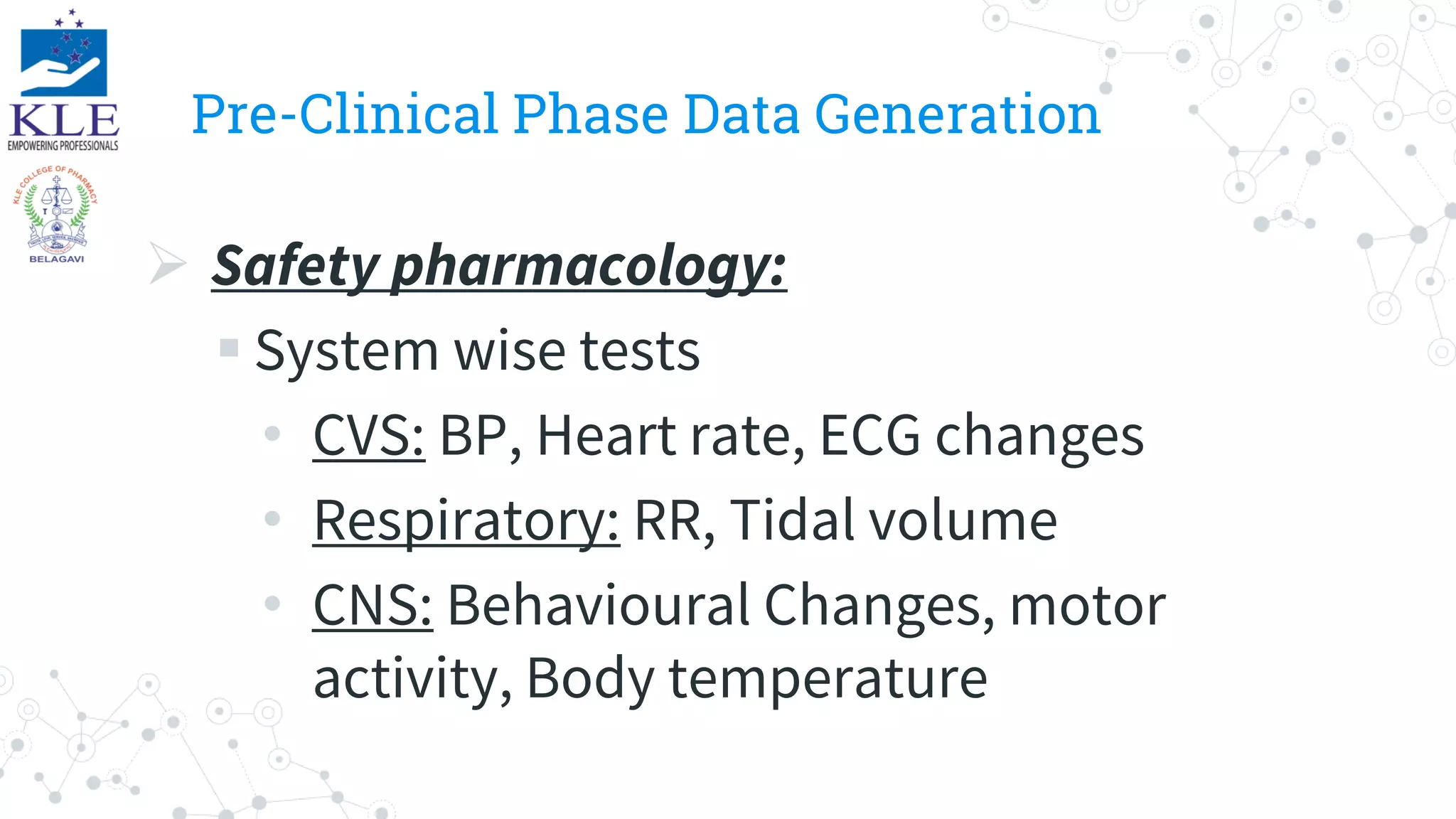
Safety data is generated throughout the drug development and approval process, including pre-clinical trials, clinical trials, and post-approval monitoring. In pre-clinical trials, exploratory and regulatory toxicology studies provide safety data. Clinical trials involve monitoring adverse events and serious adverse events. Post-approval, safety is monitored through periodic safety update reports, spontaneous reporting, and other methods. Safety data informs the entire process from drug discovery through marketing.