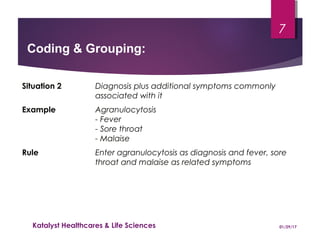
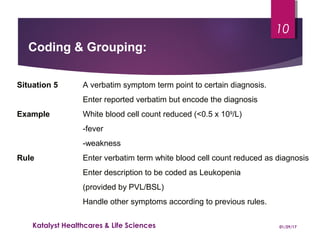
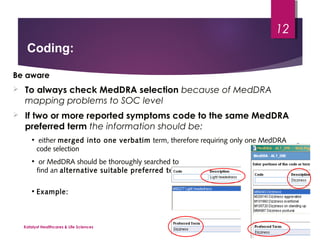
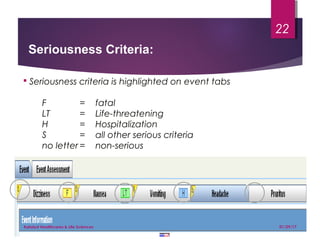
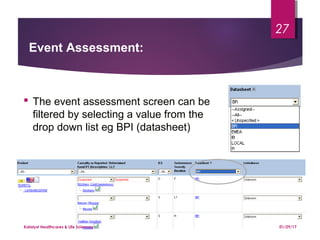
The document outlines coding and grouping standards for medical event reporting, emphasizing the importance of utilizing a standardized dictionary (MedDRA) and grouping related symptoms for accurate assessments. It provides examples of various situations to effectively code symptoms and diagnoses, including rules for handling multiple diagnoses, entering consequences of leading events, and dealing with adverse events. Additionally, it includes guidelines on event assessment, seriousness criteria, and the necessary precautions in coding processes.