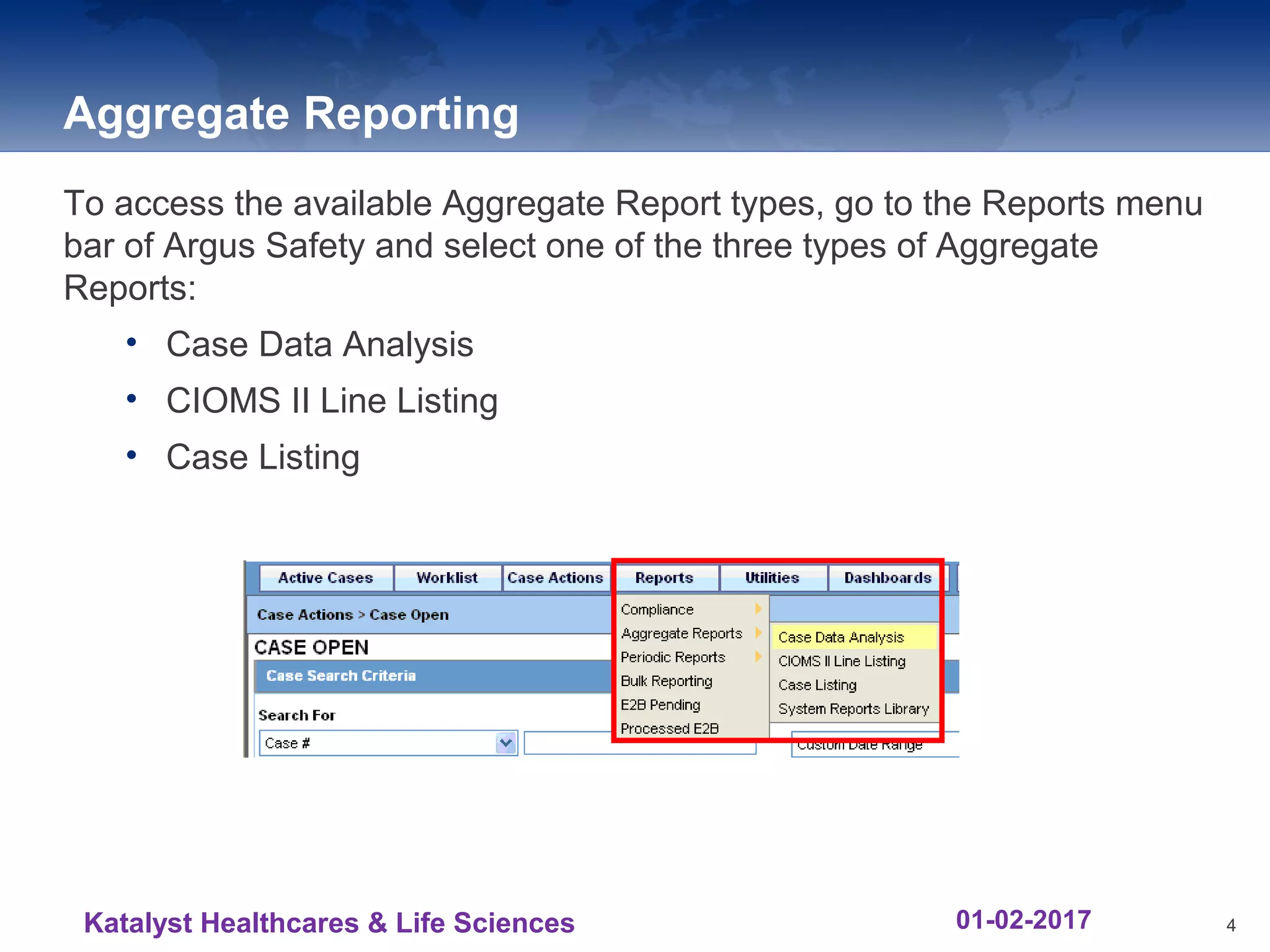
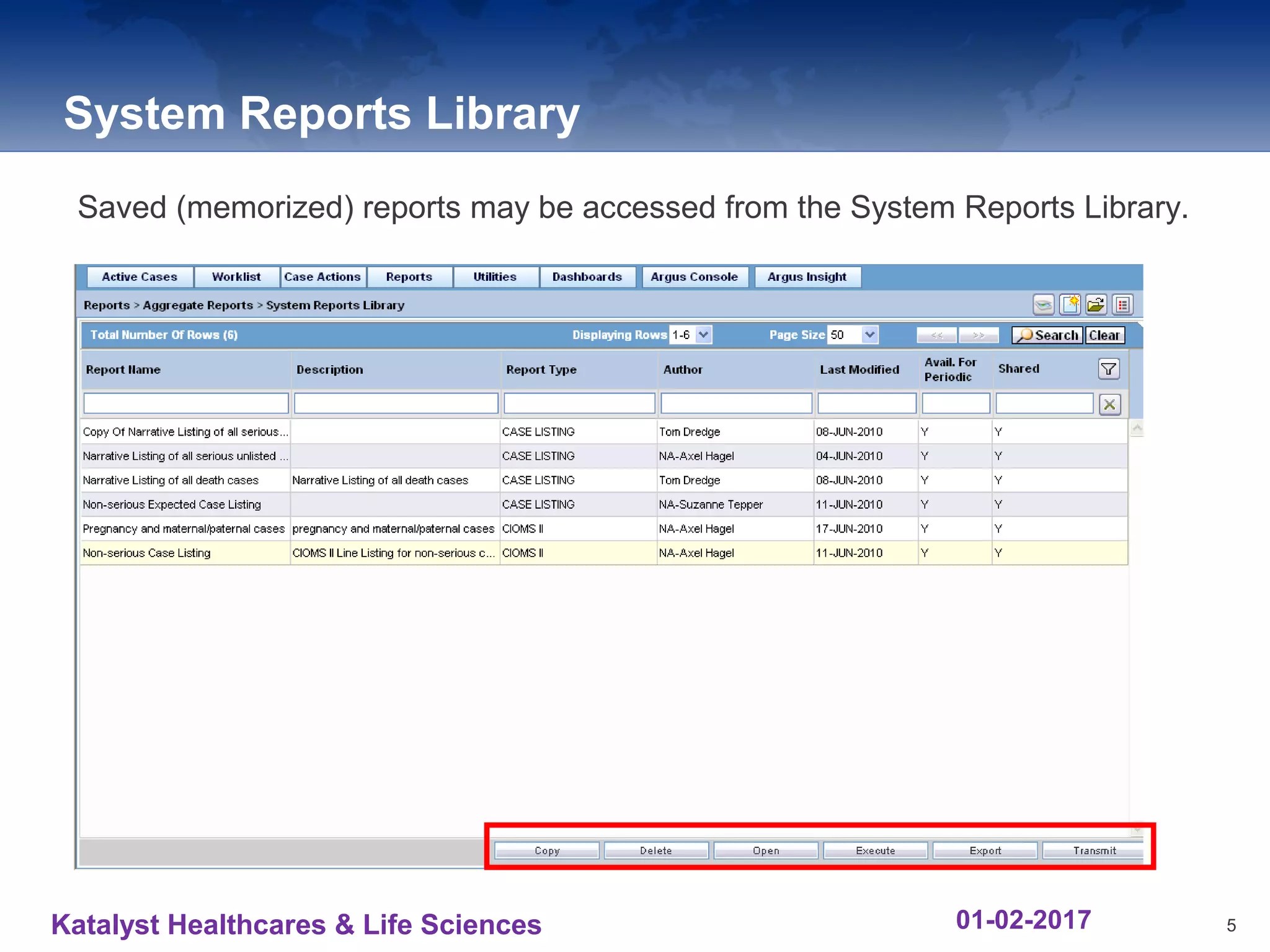
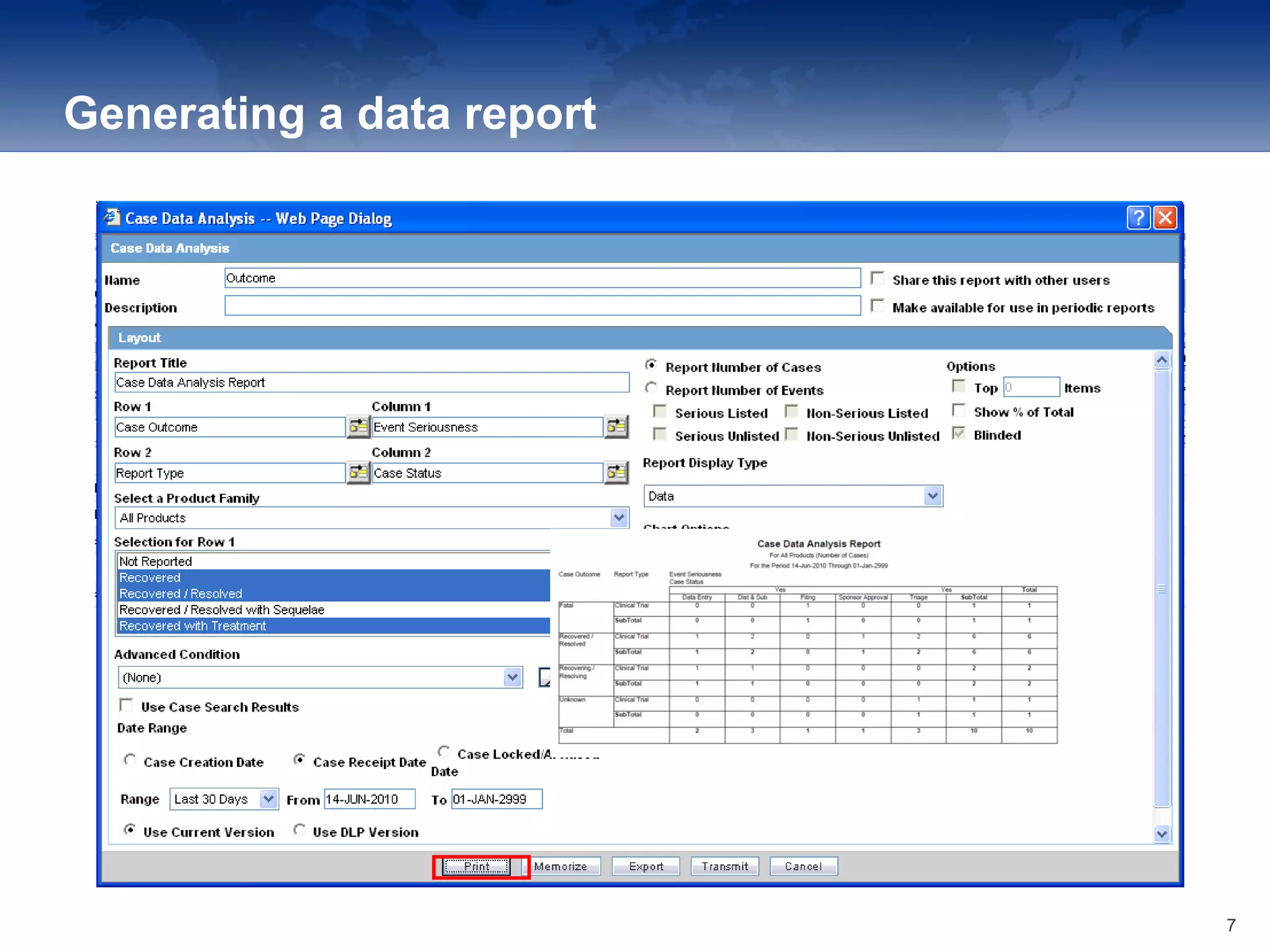
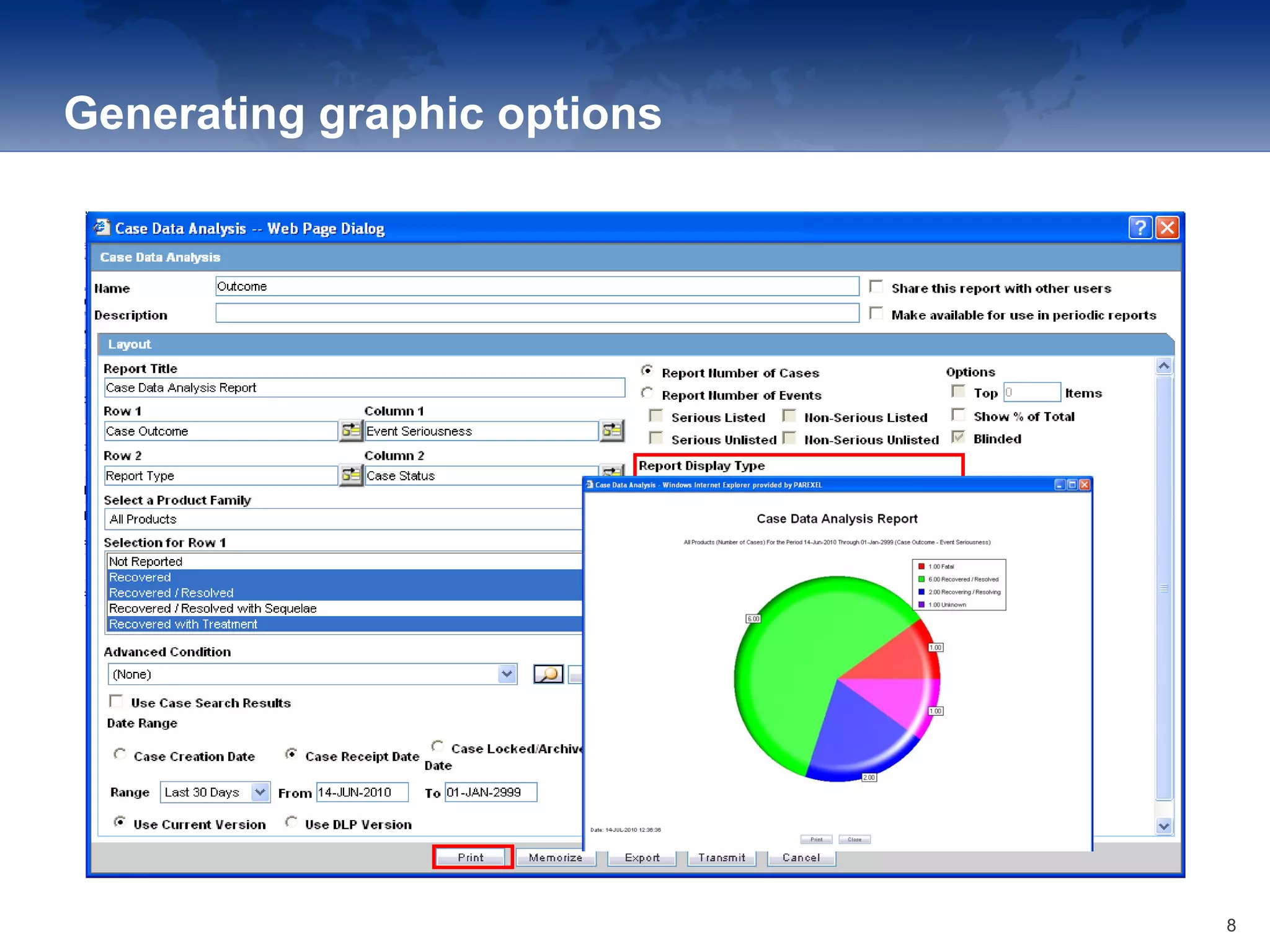
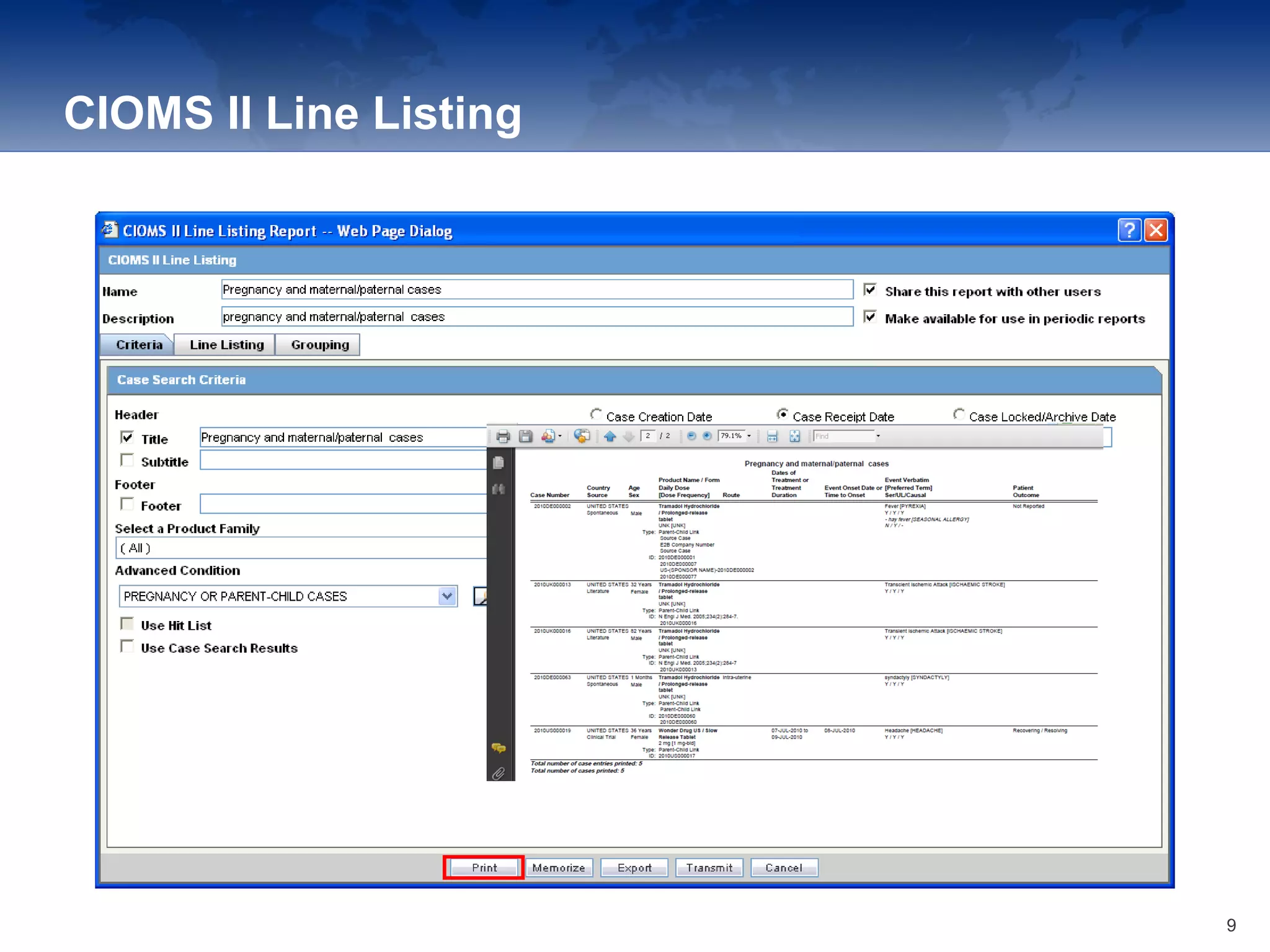
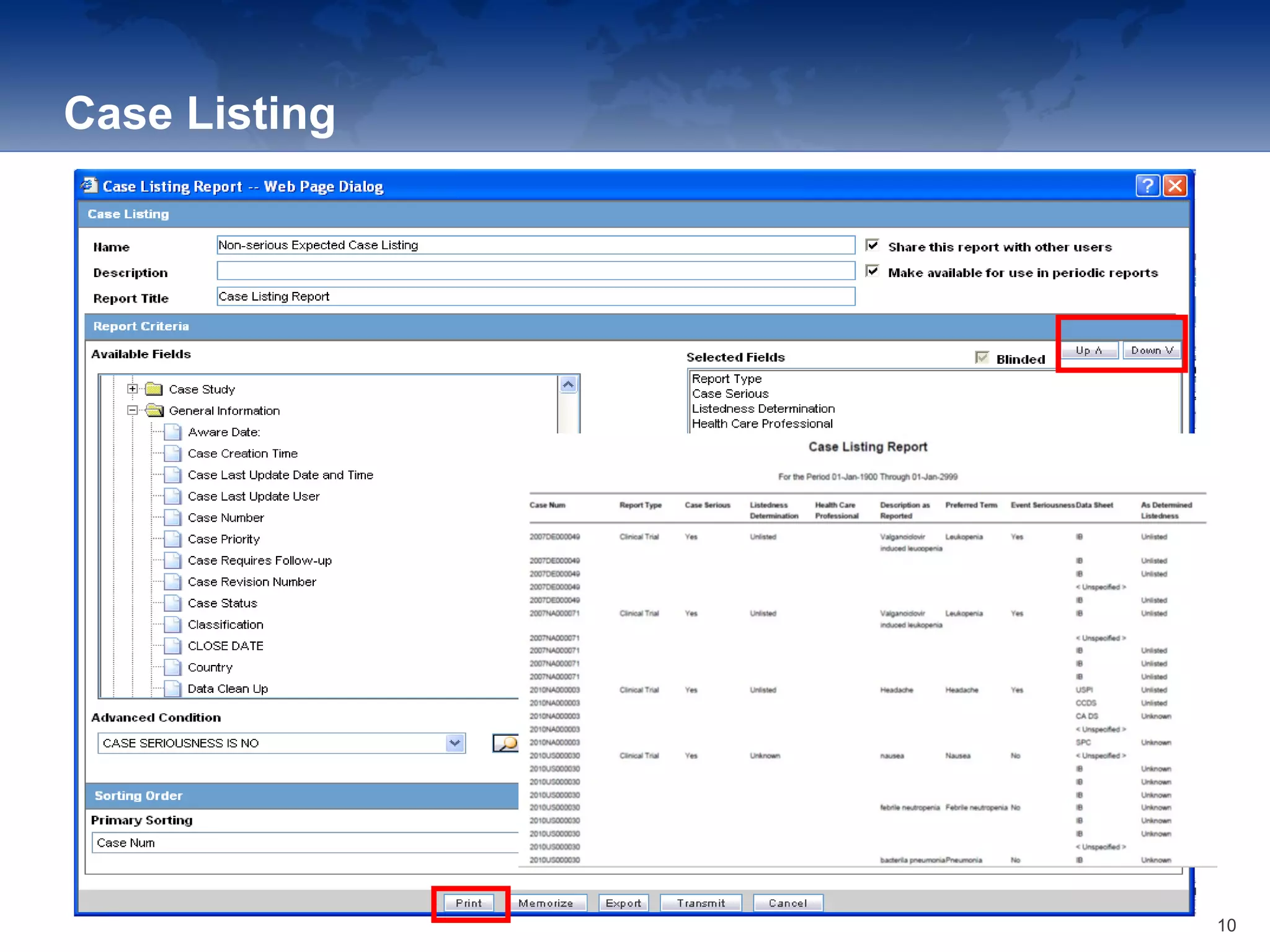
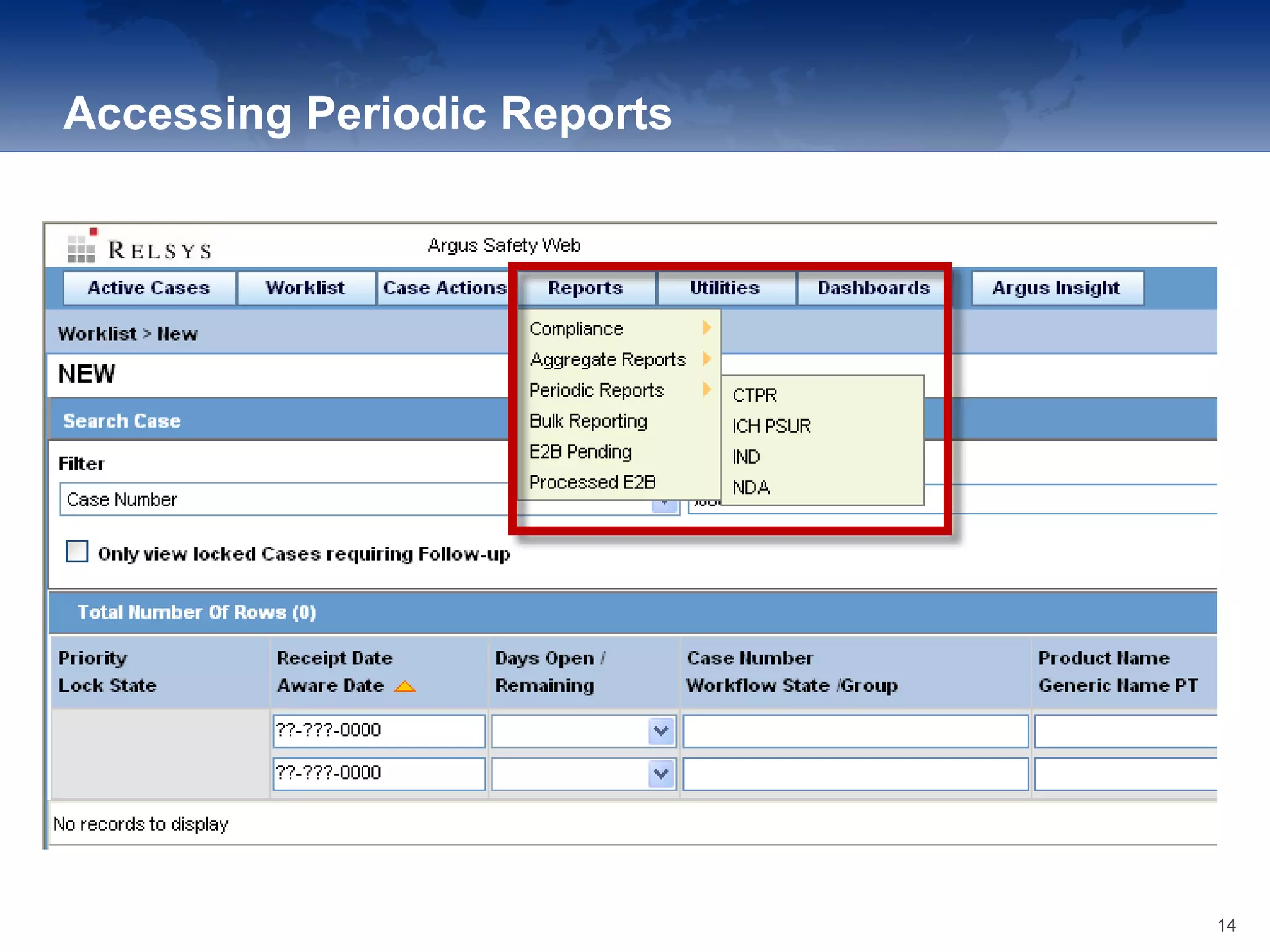
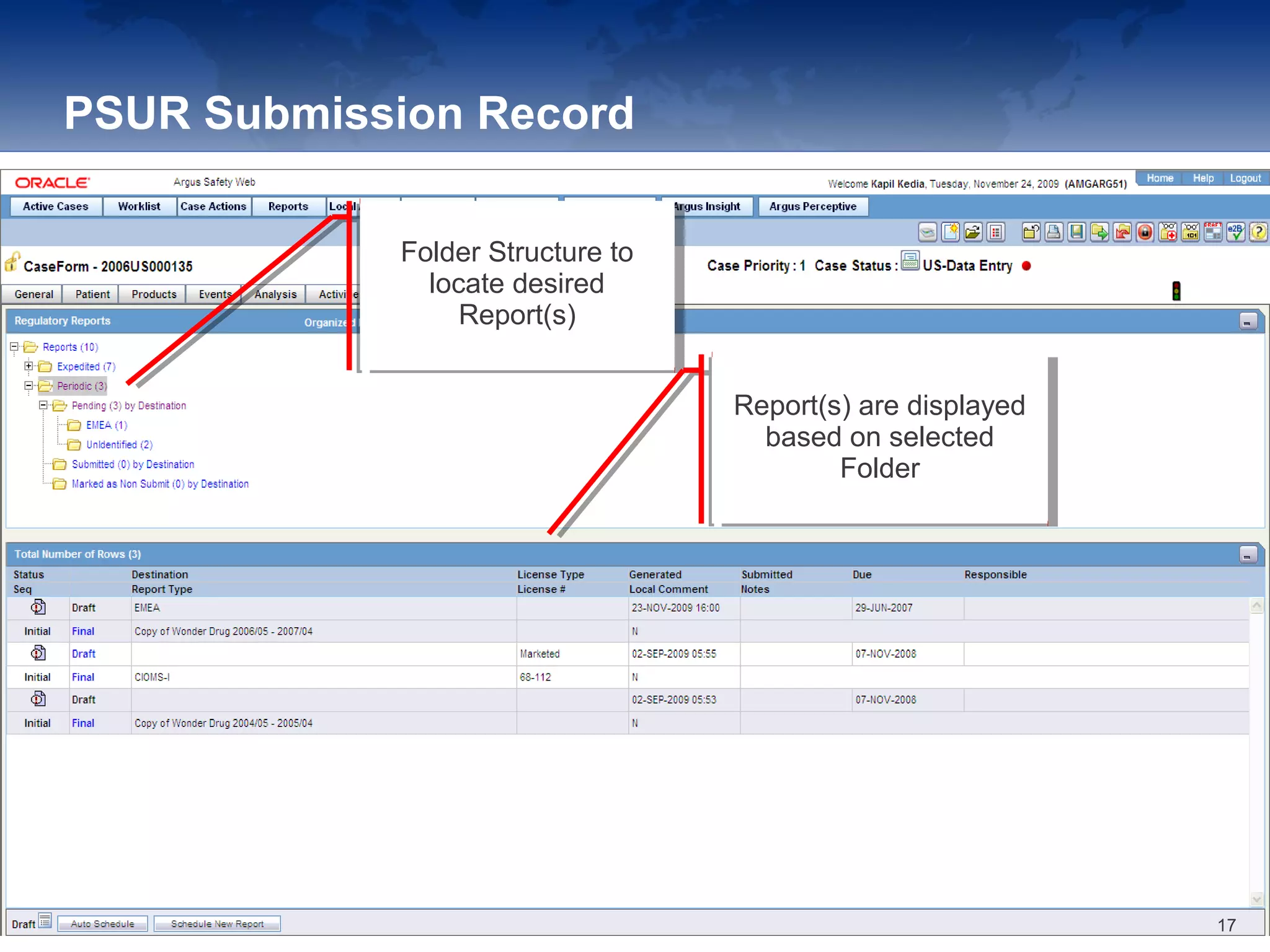
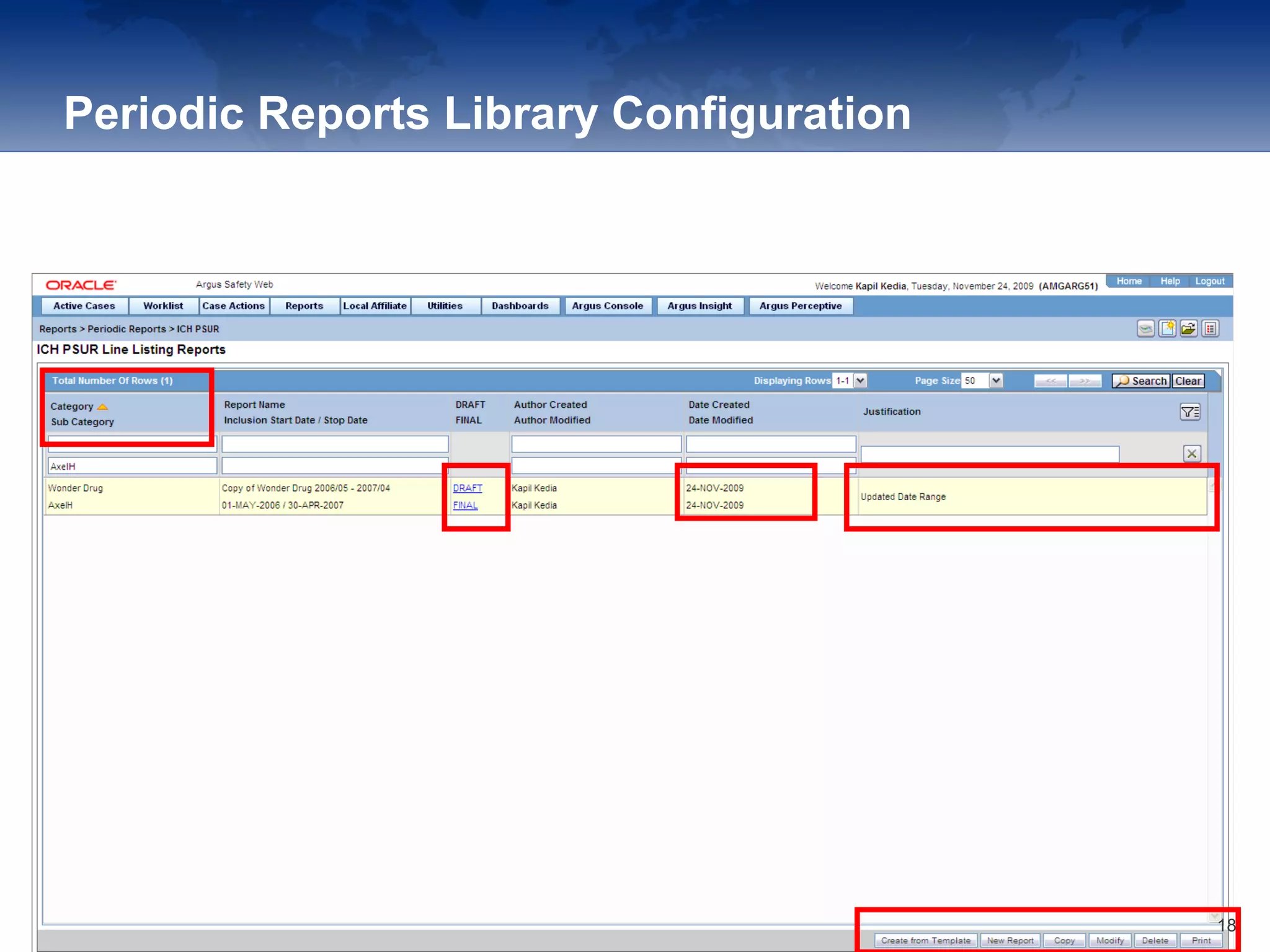
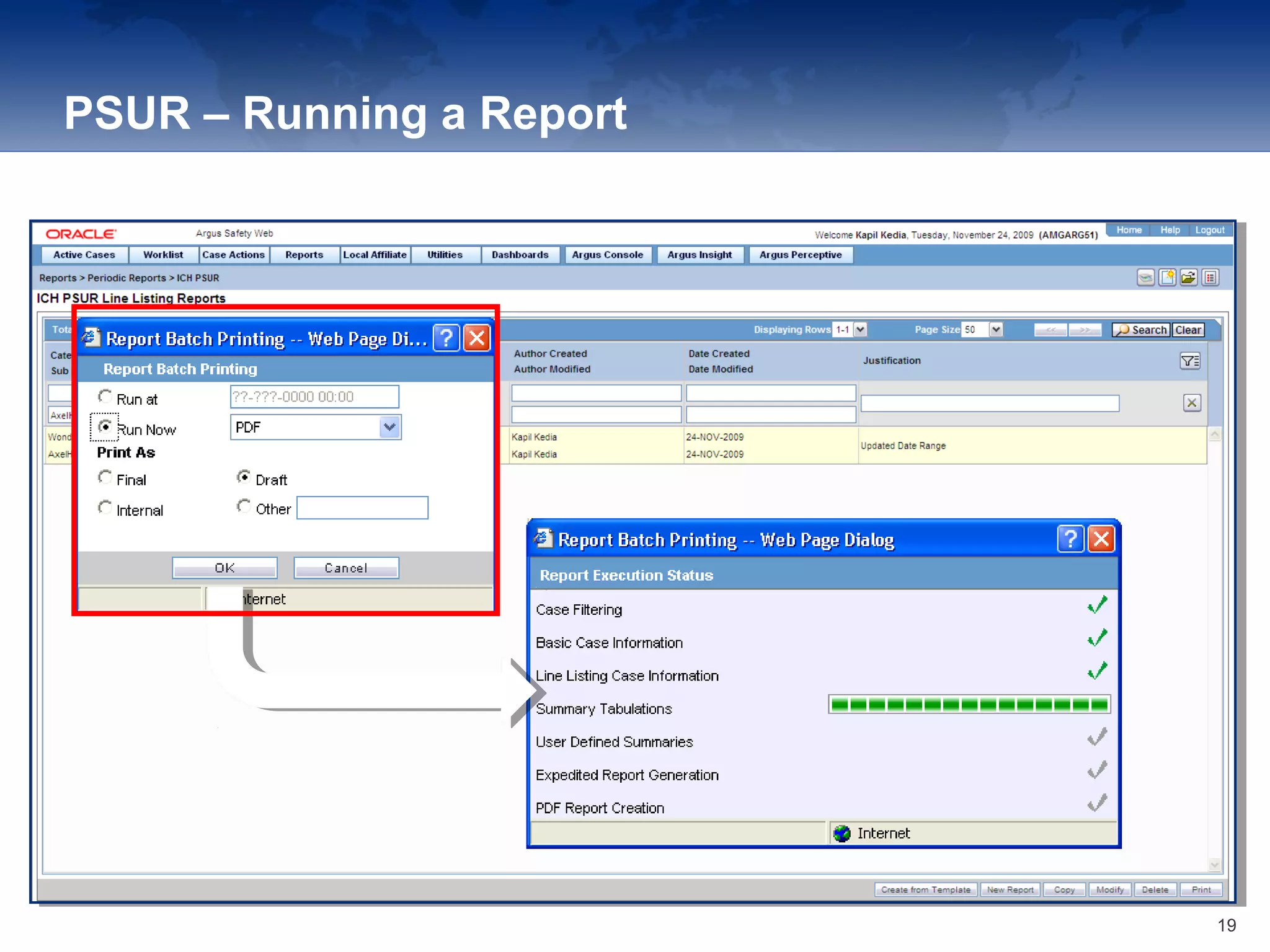
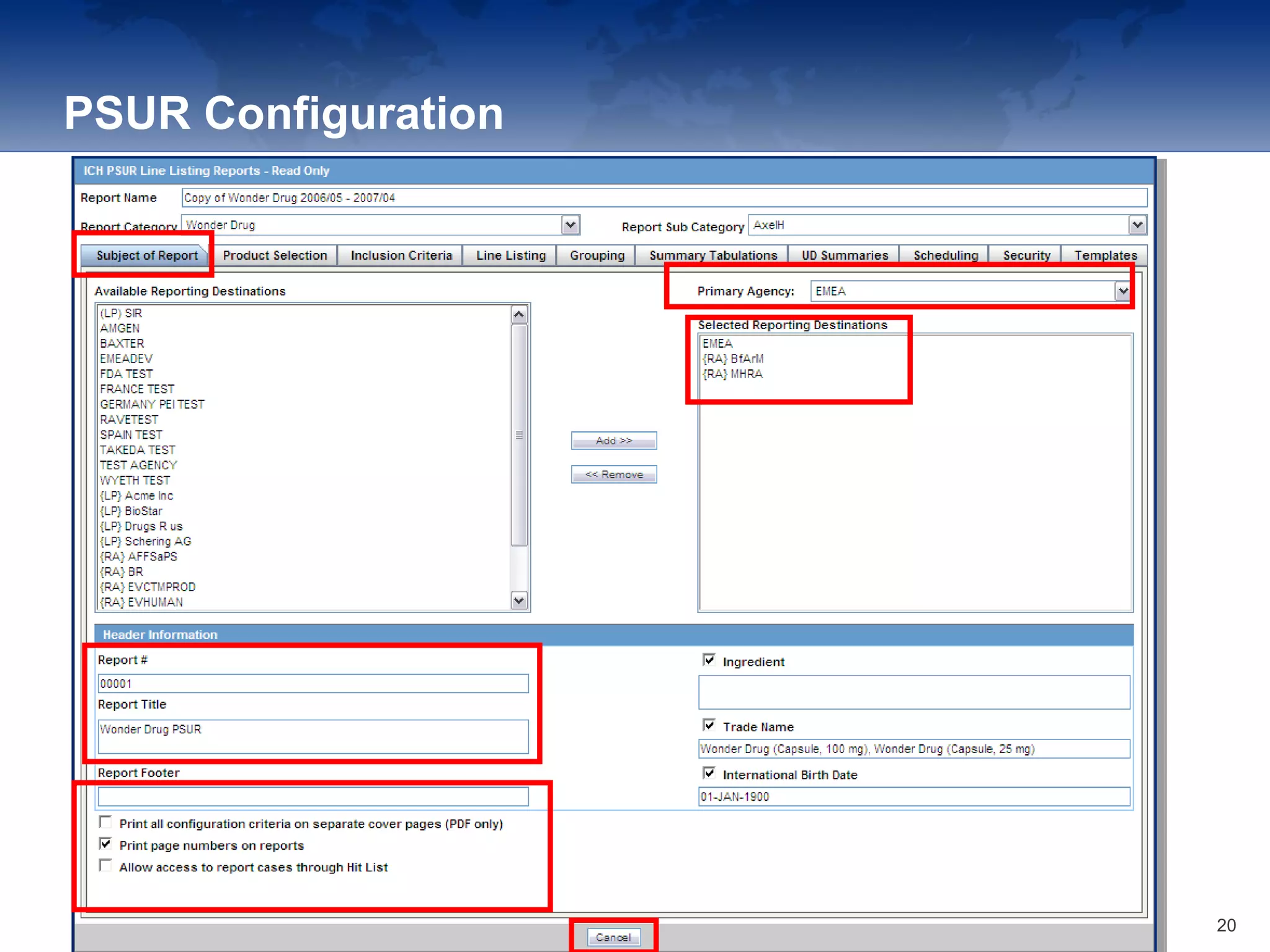
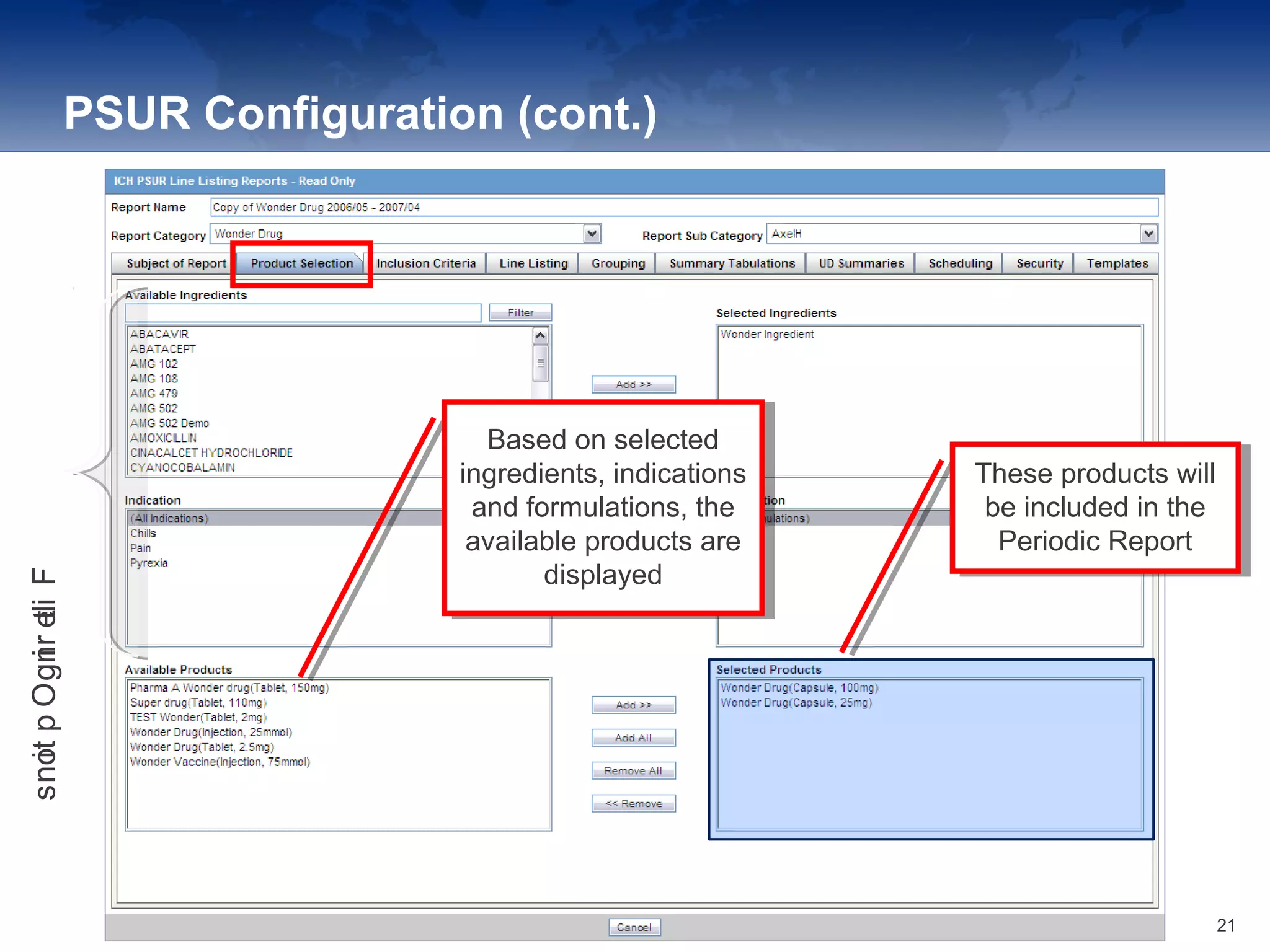
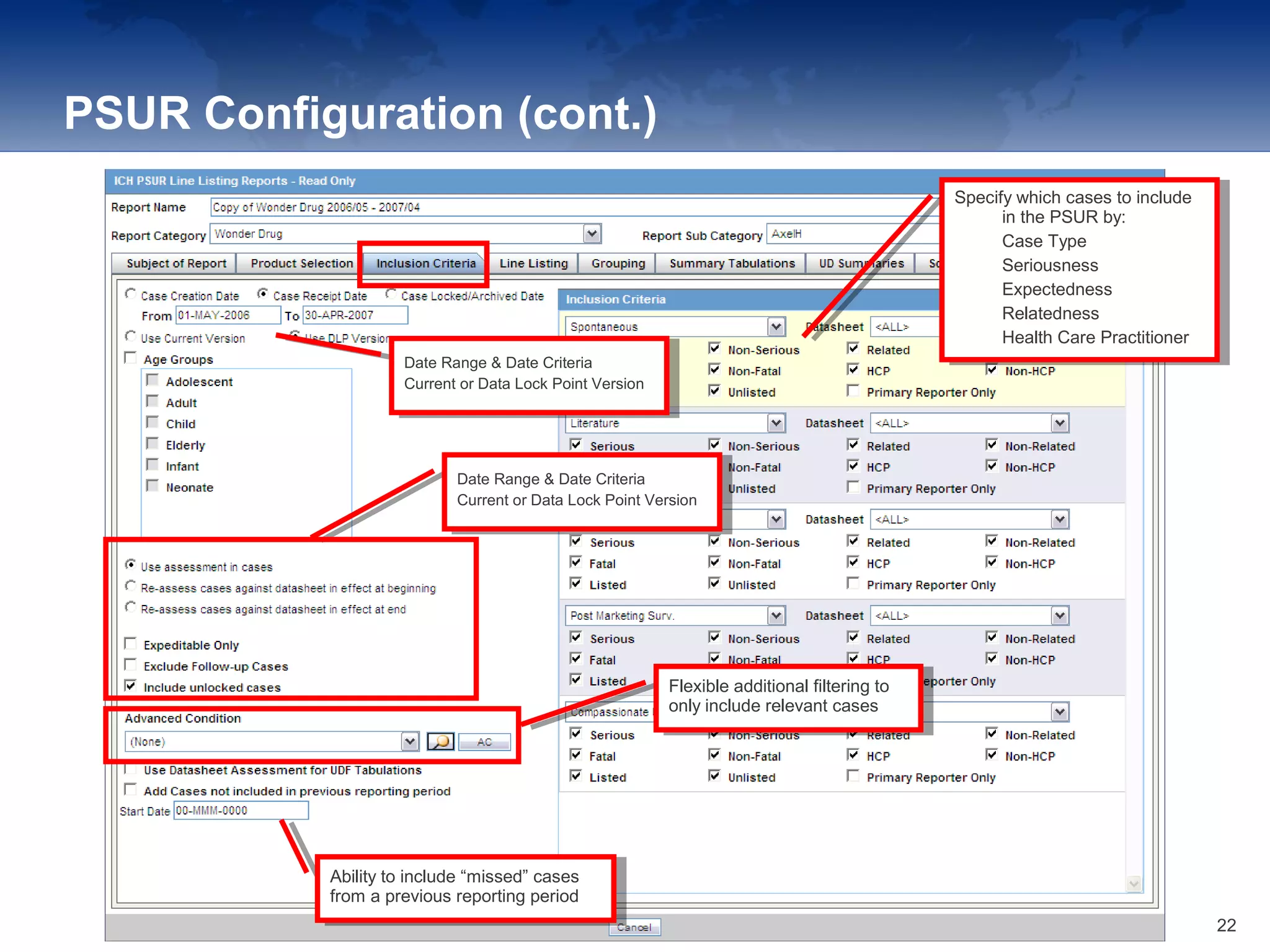
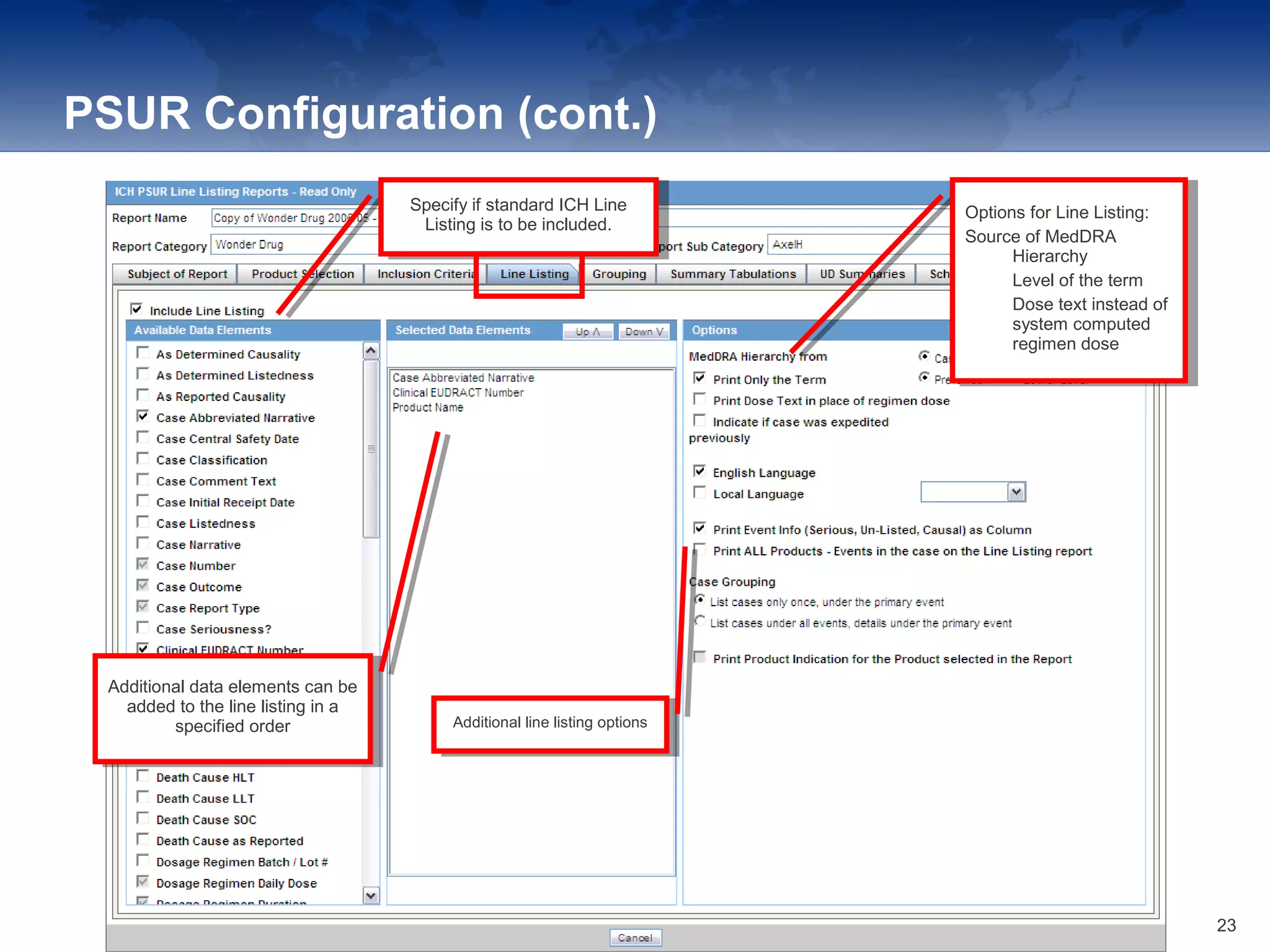
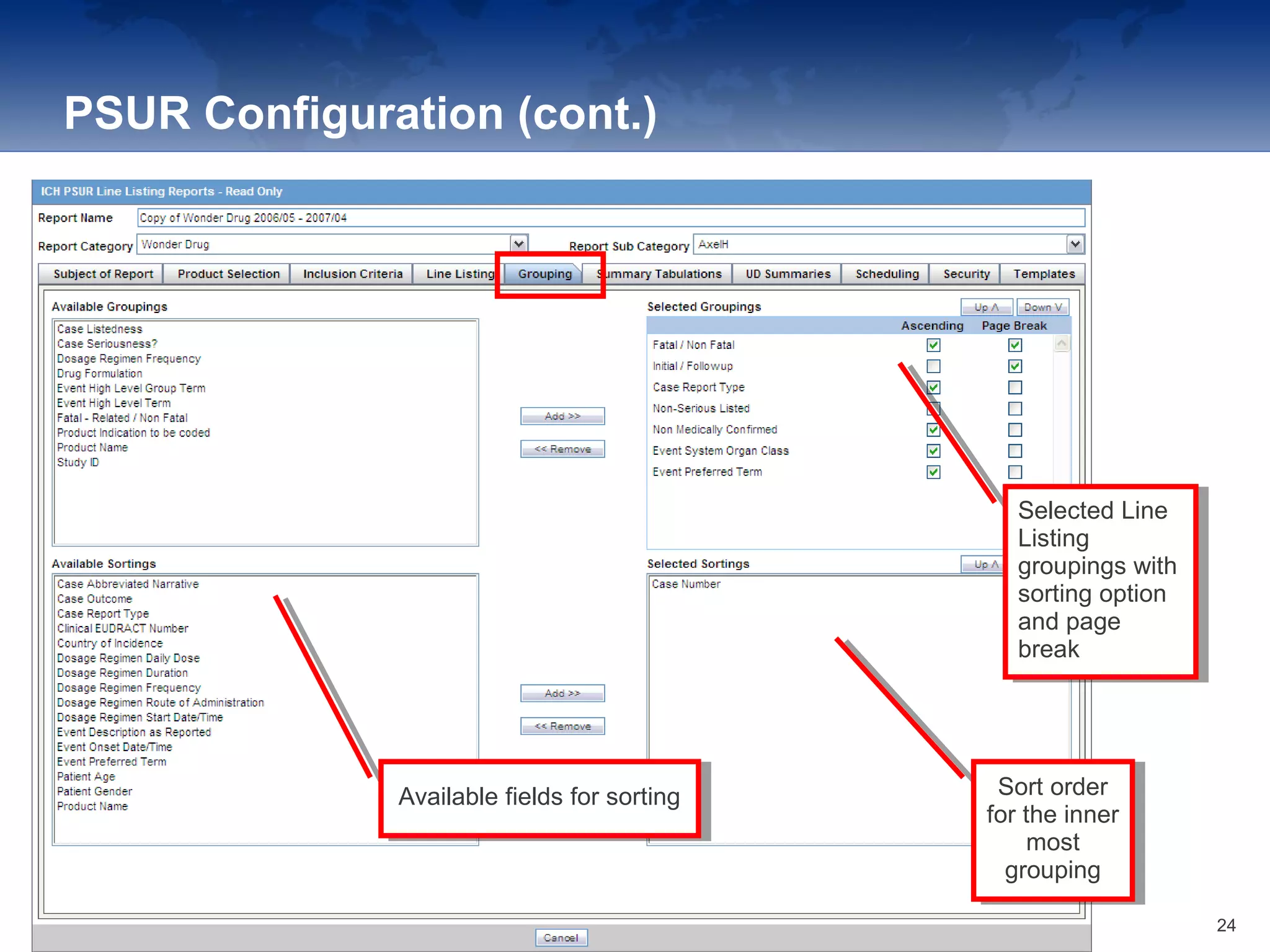
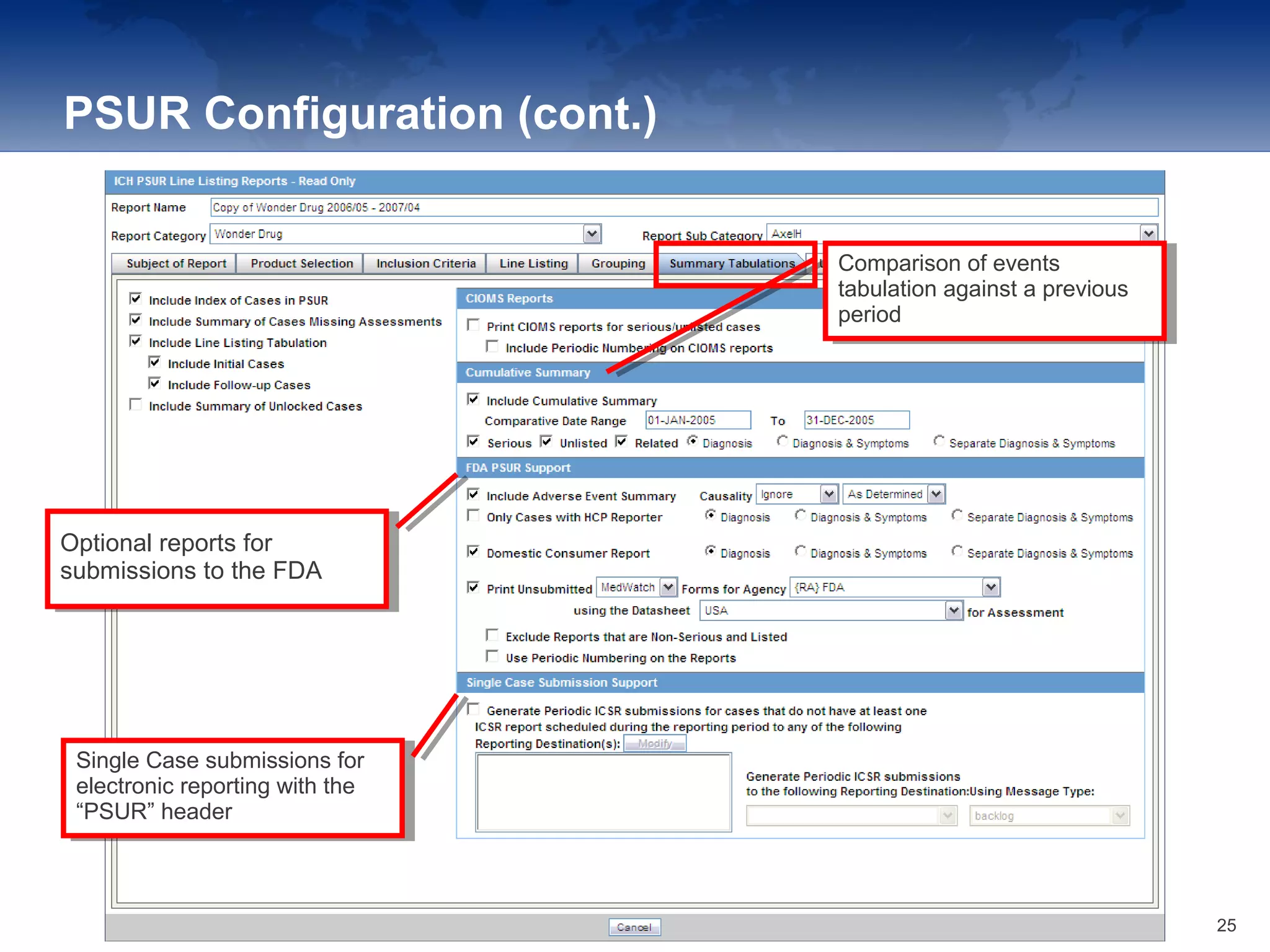
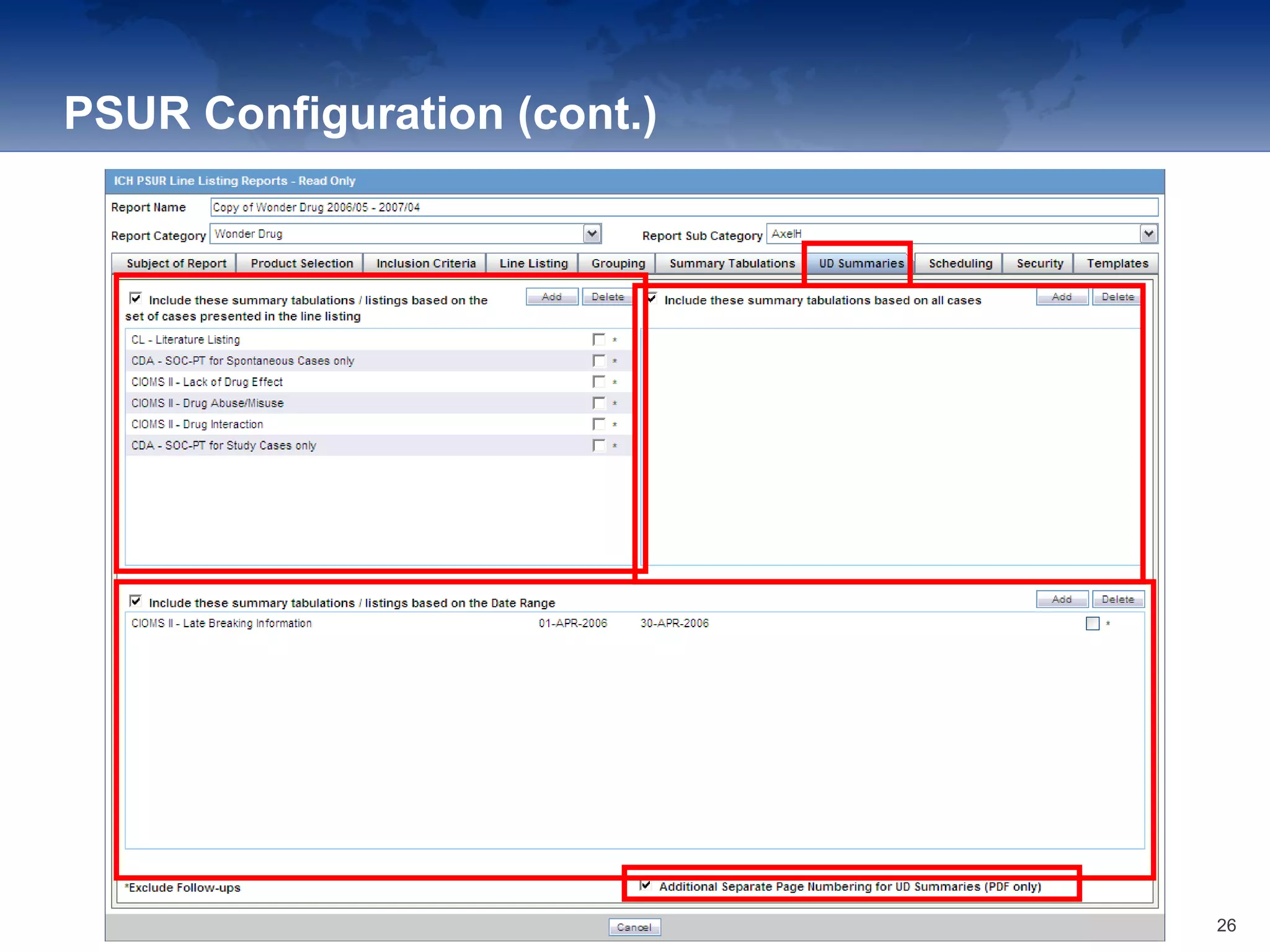
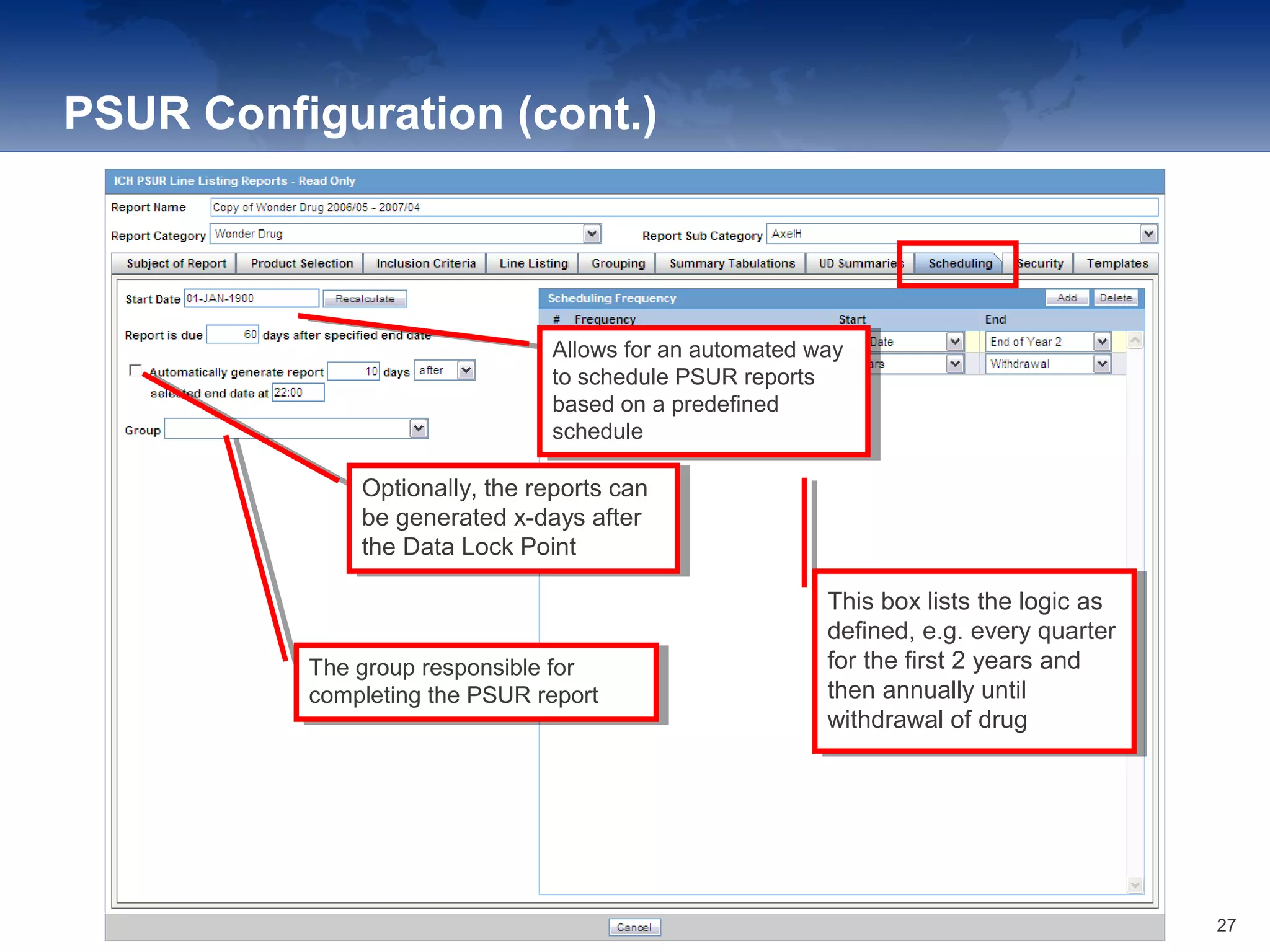
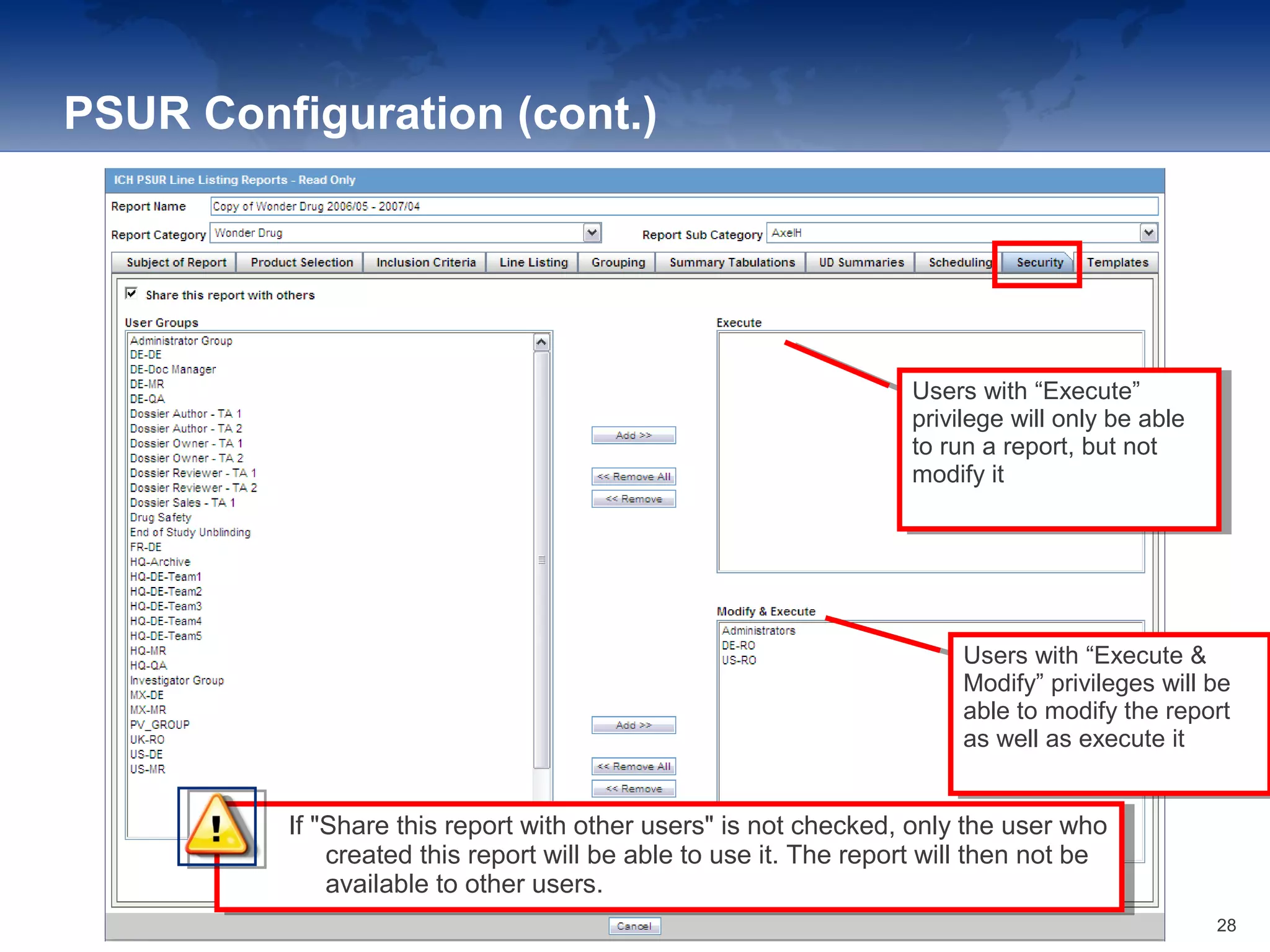
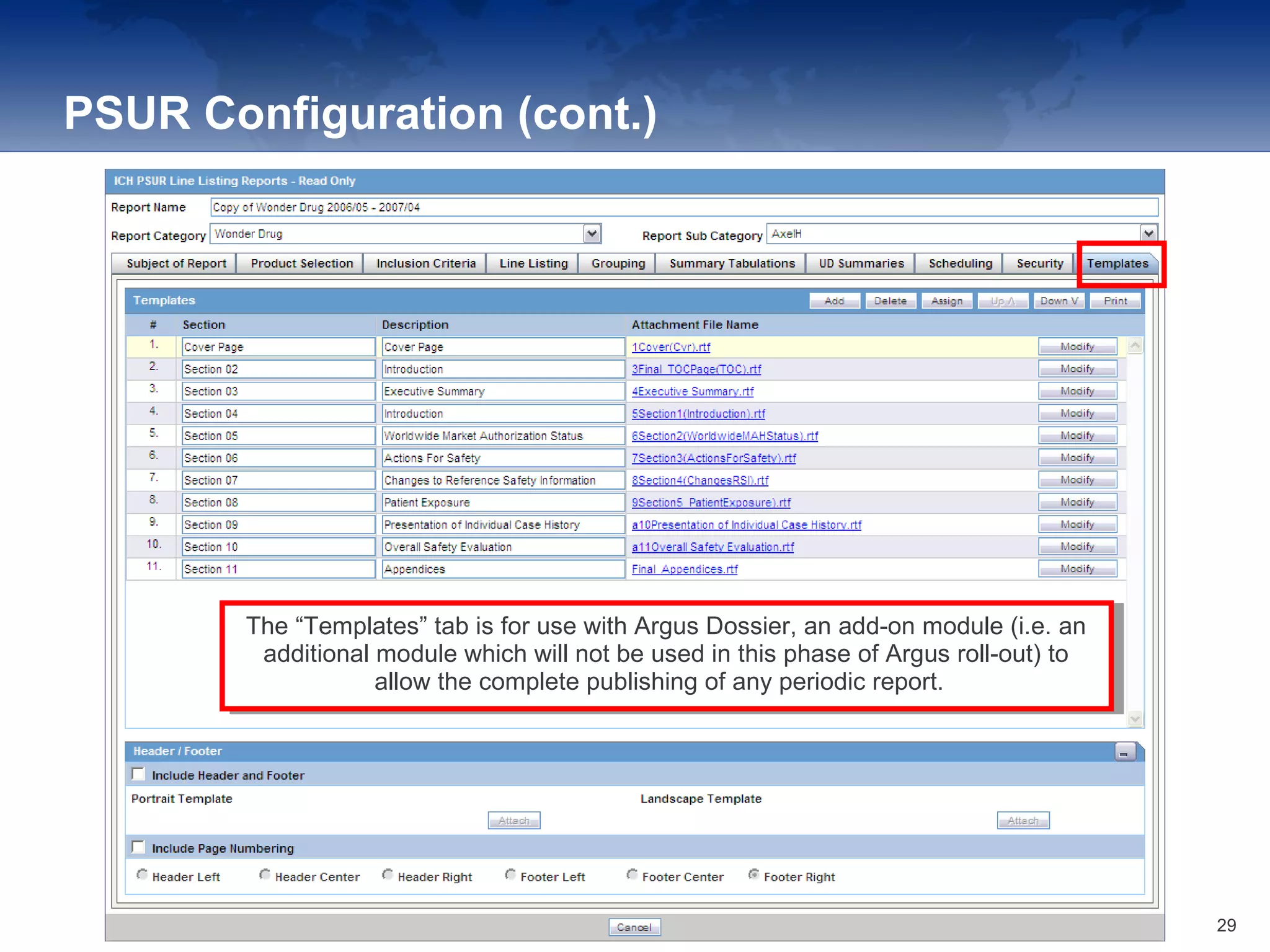
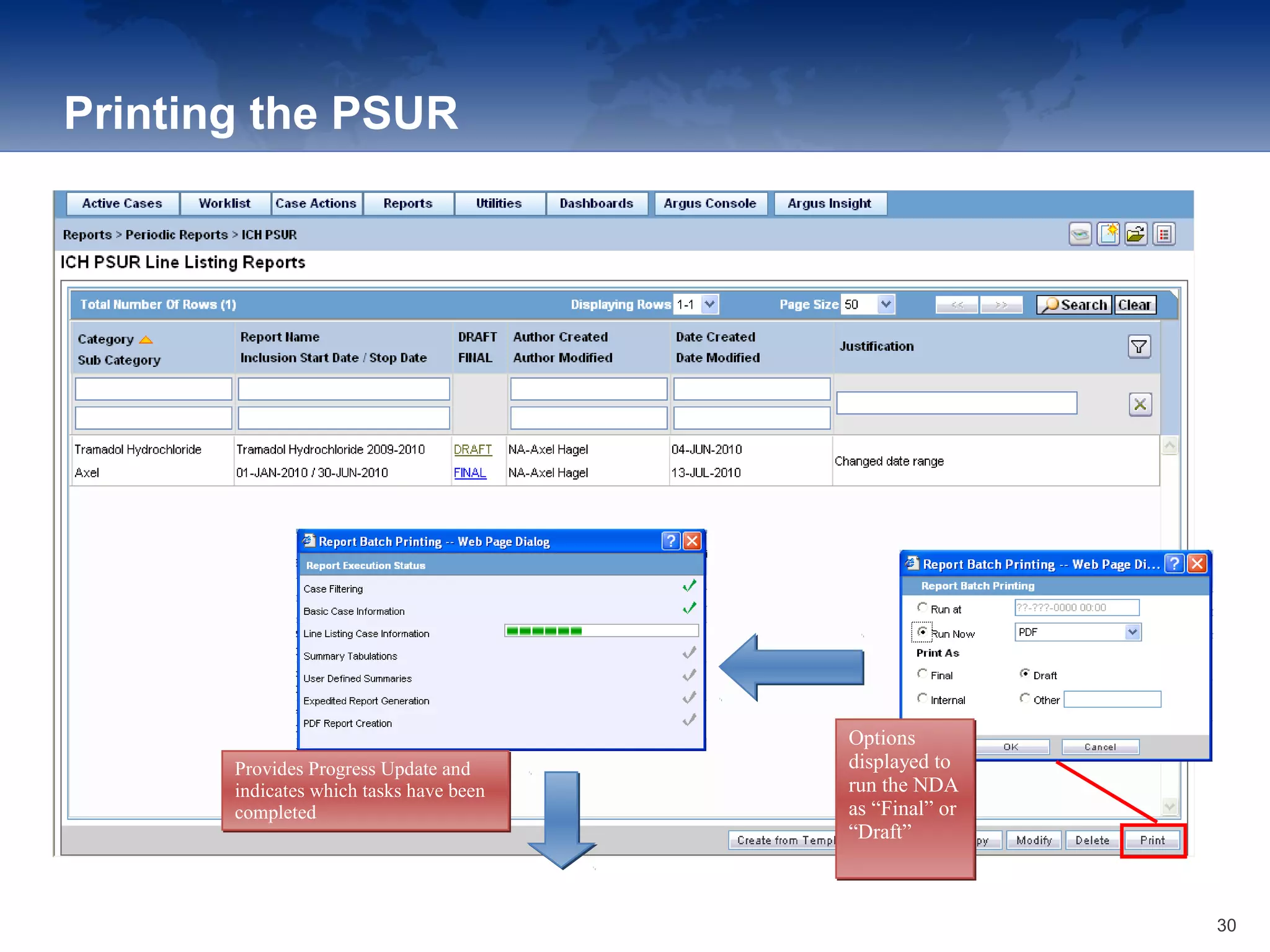
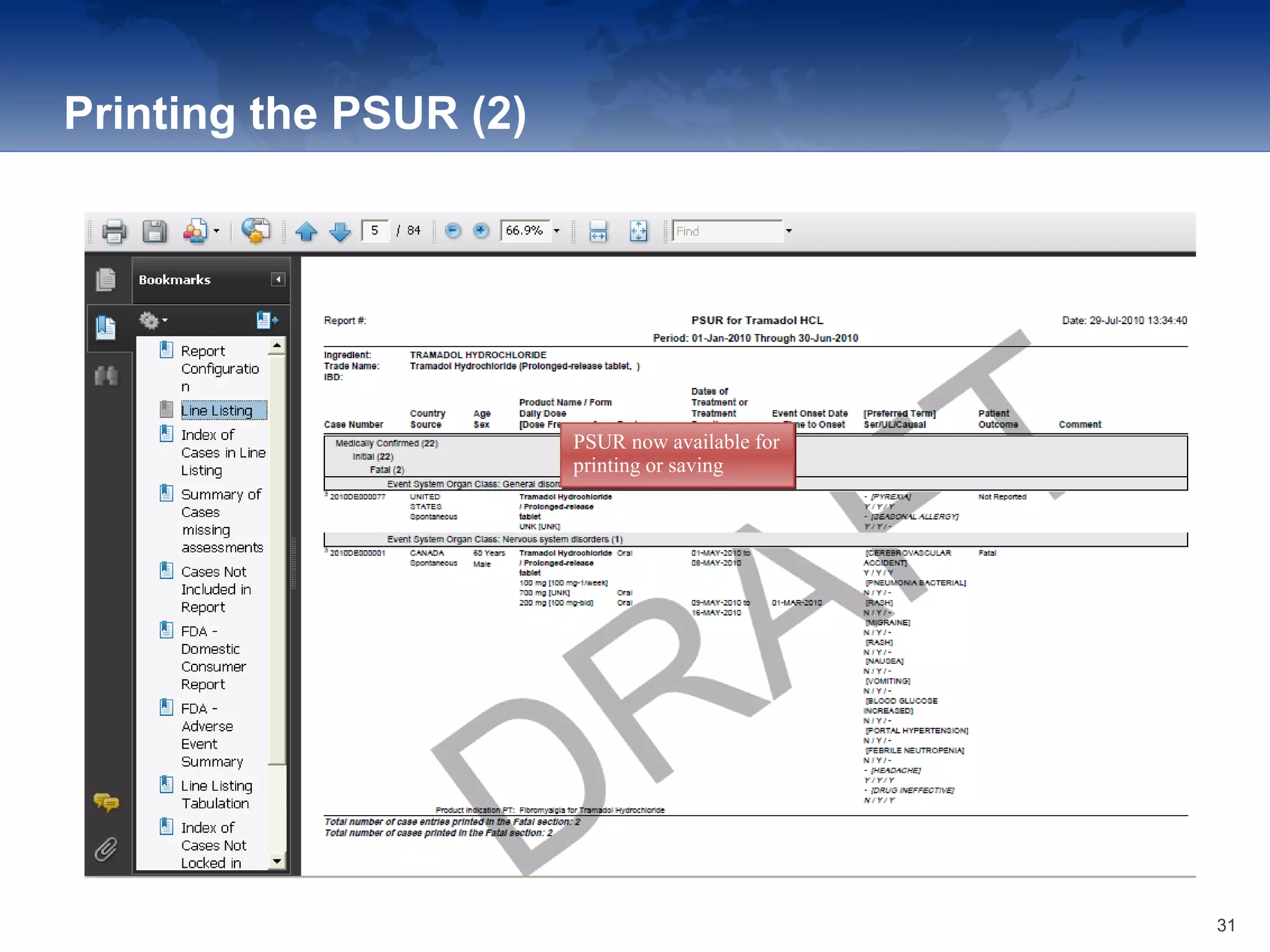
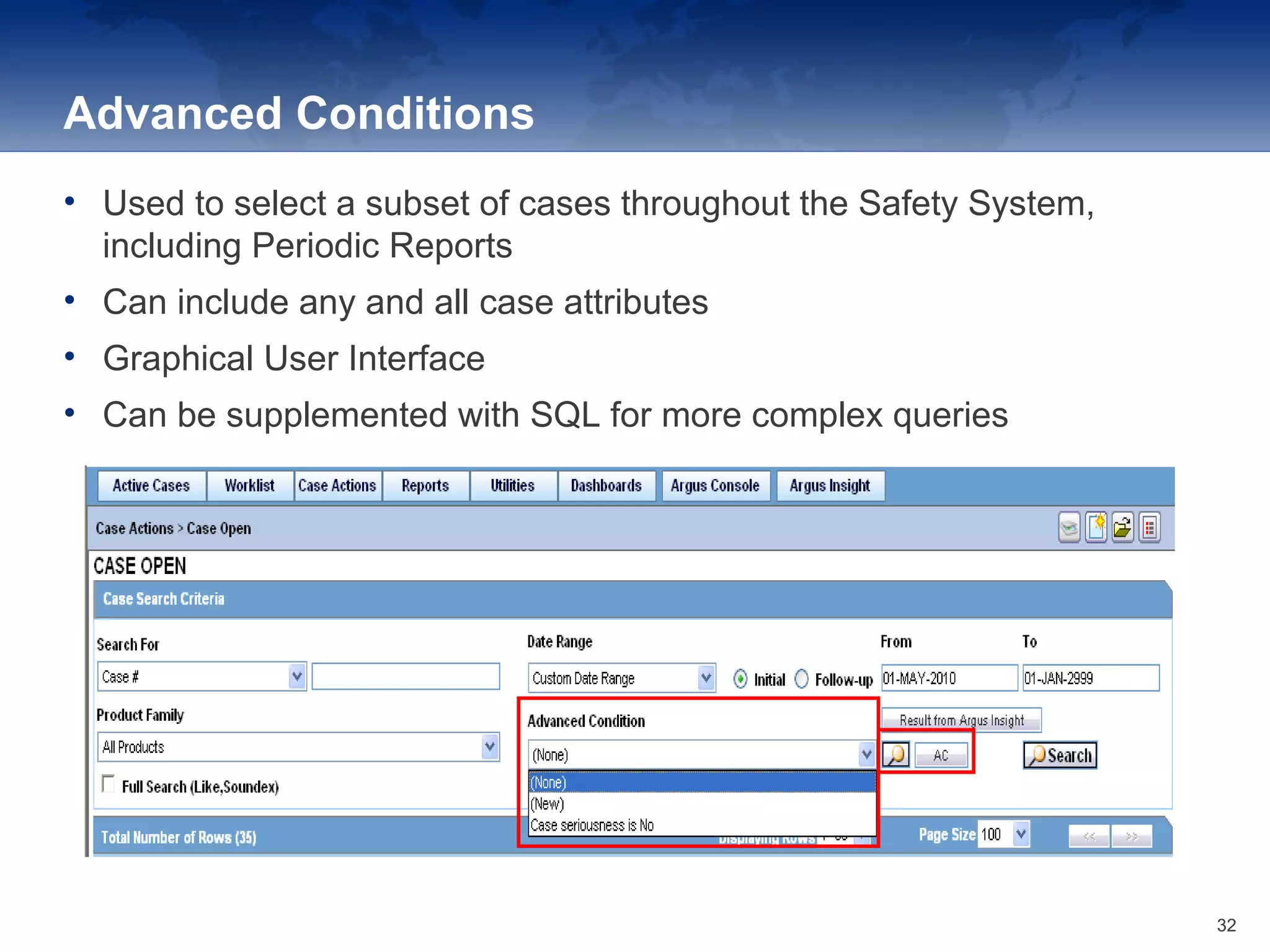
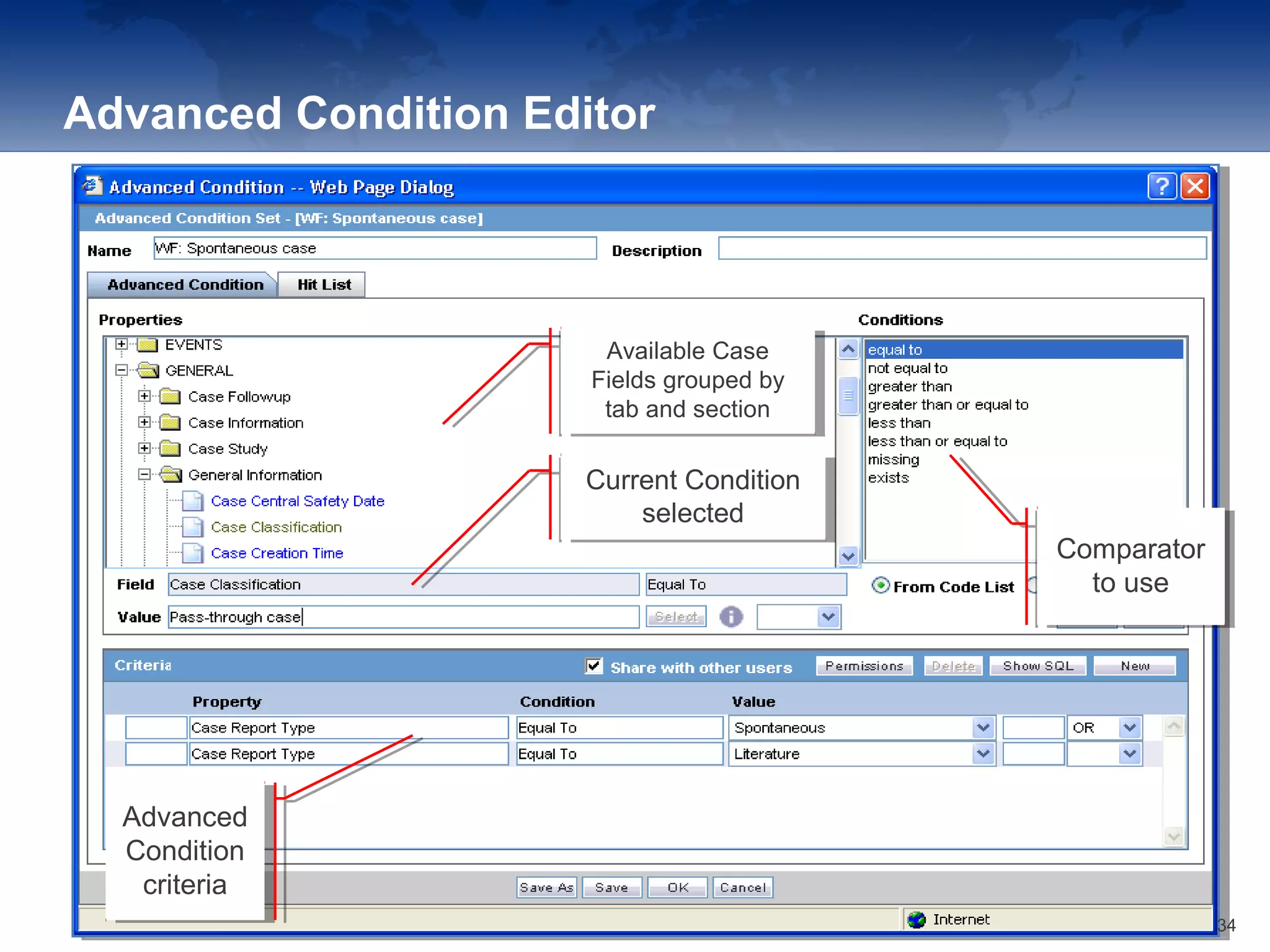
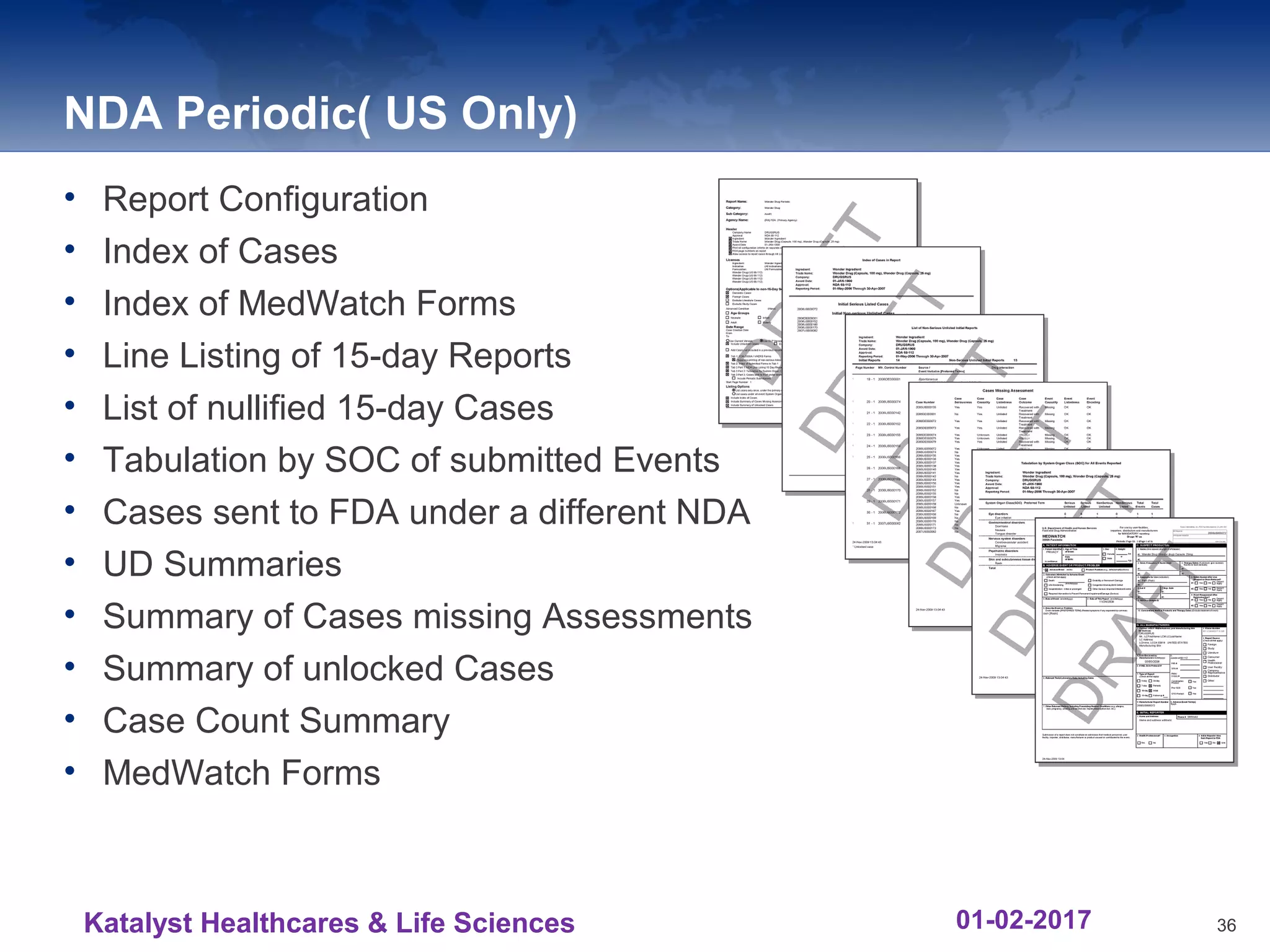
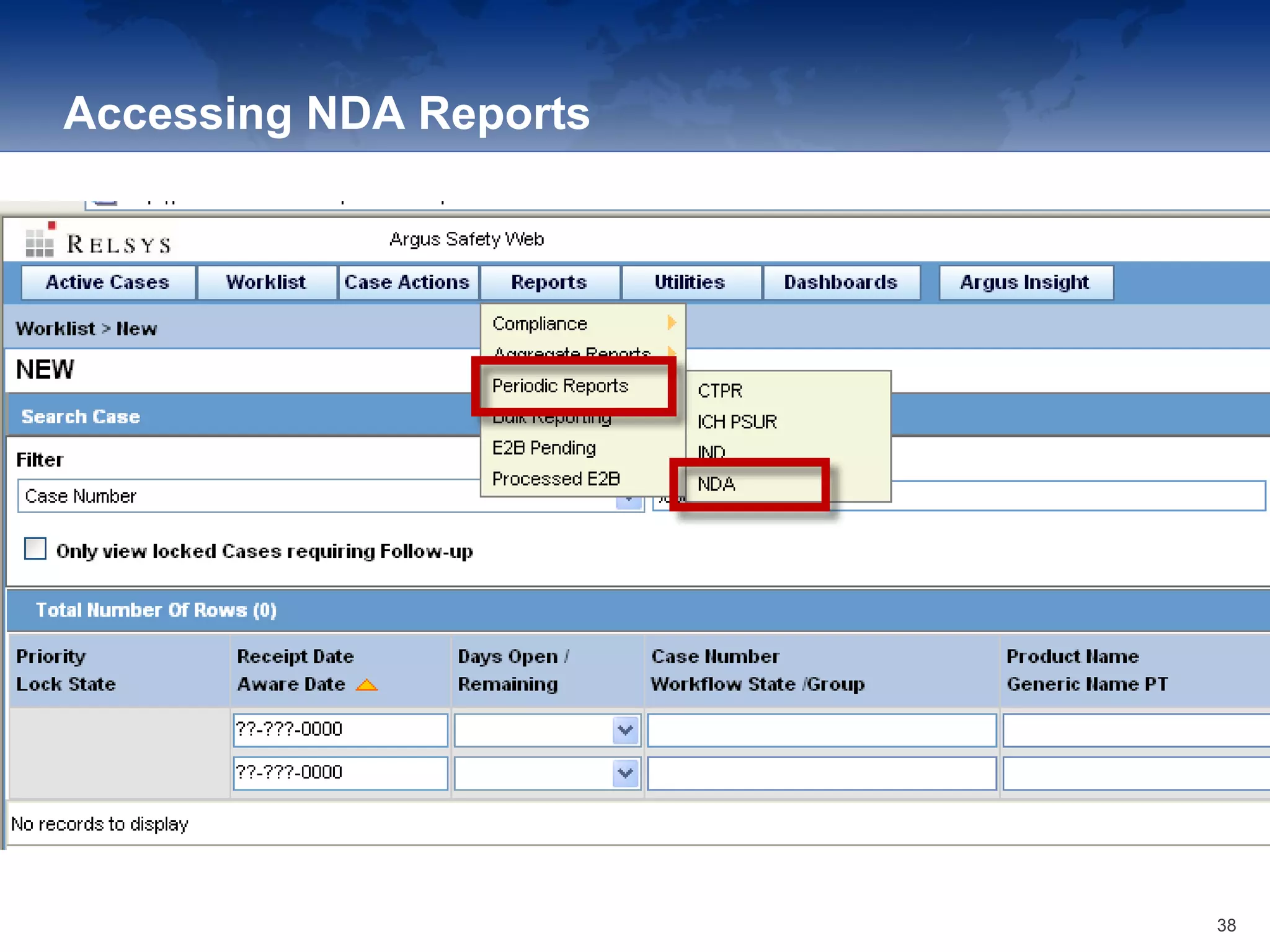
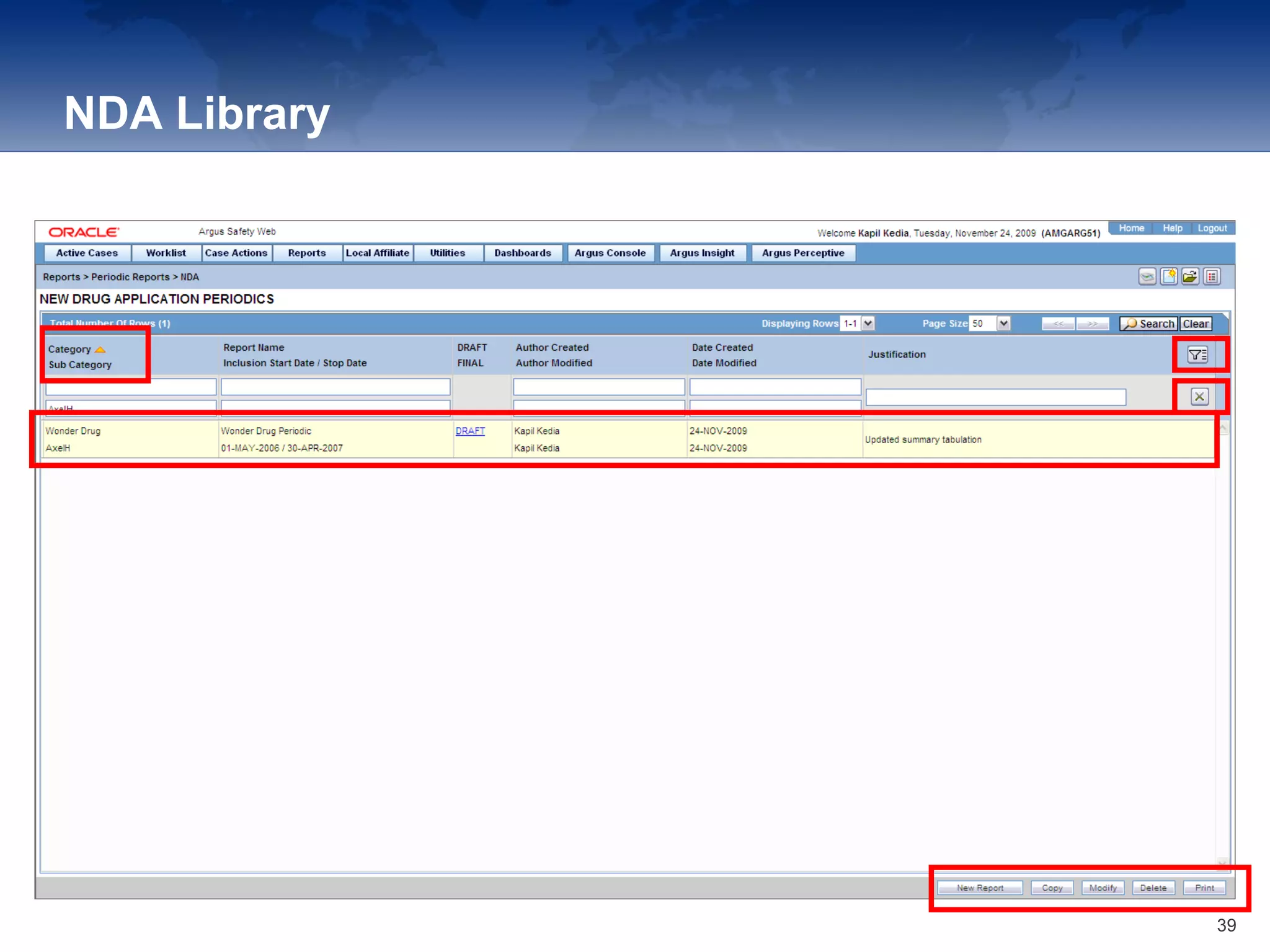
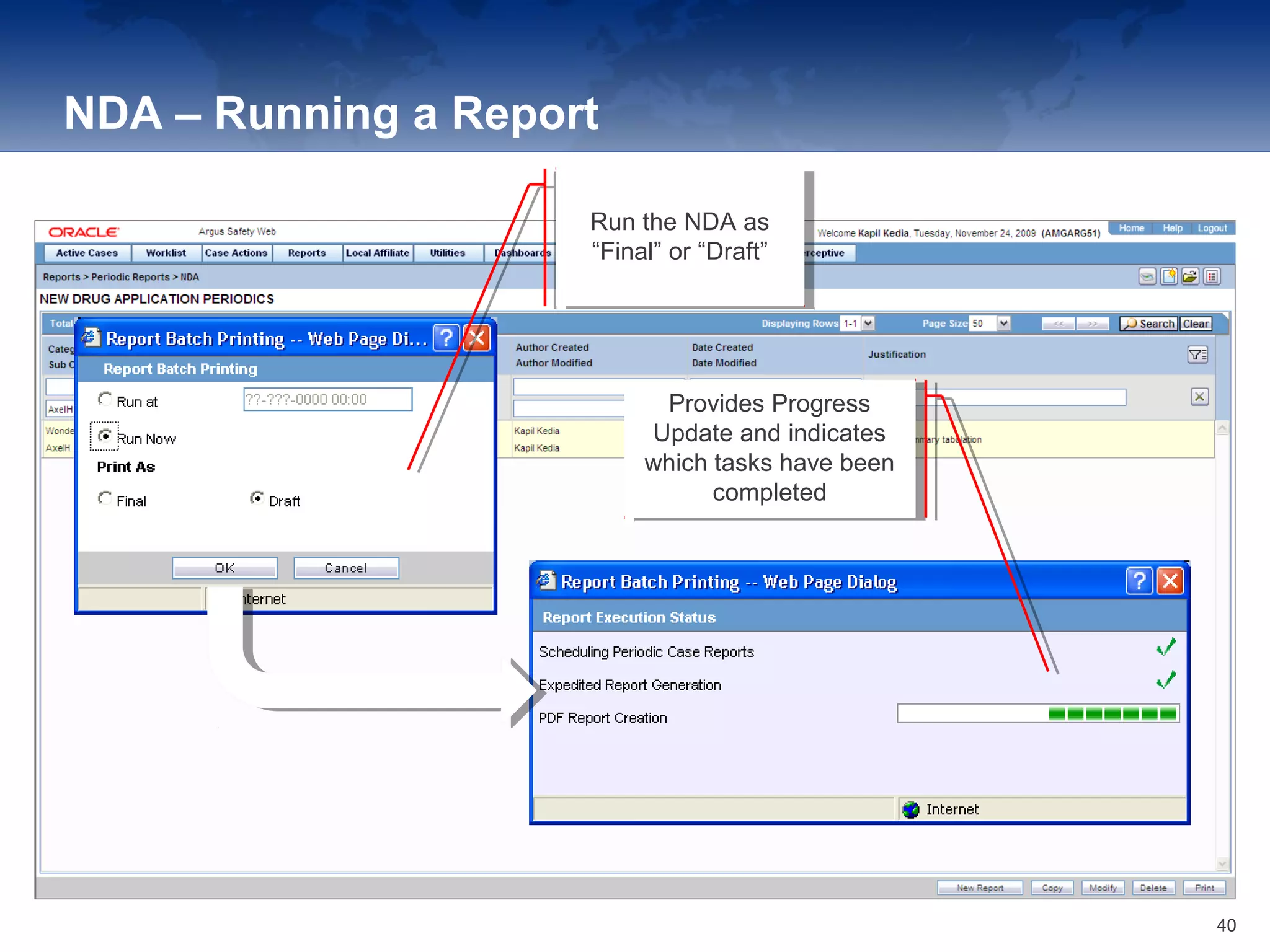
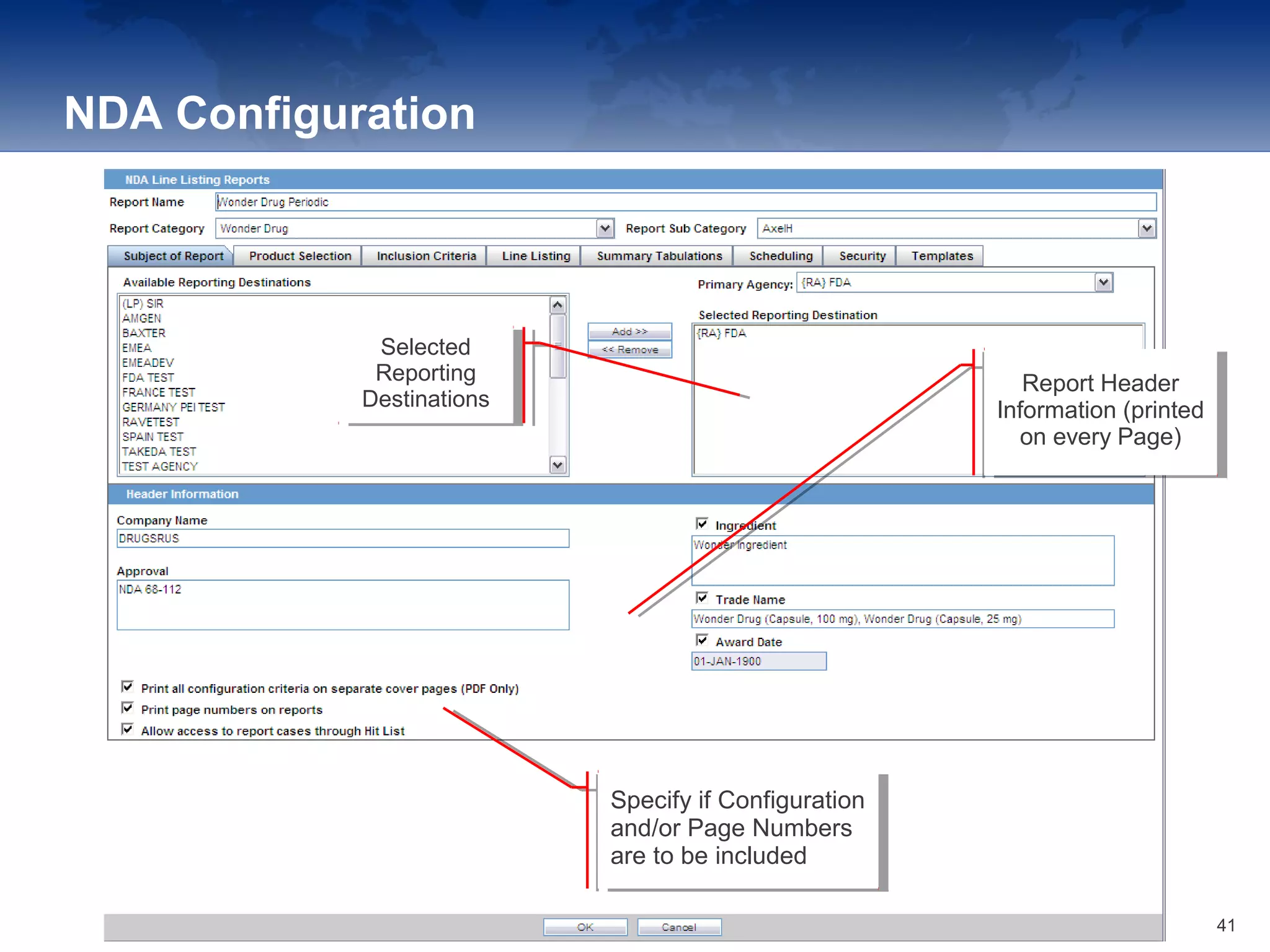
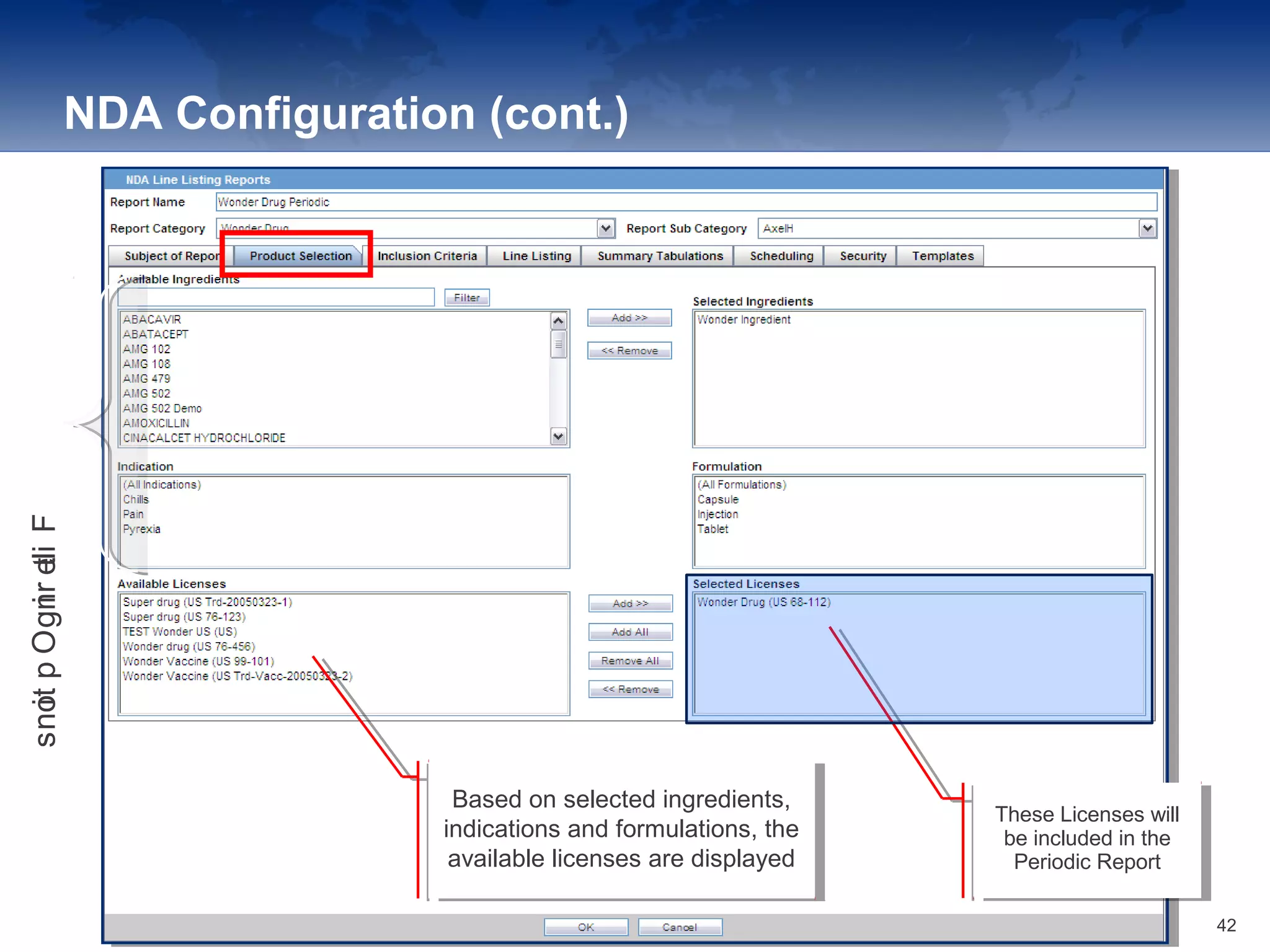
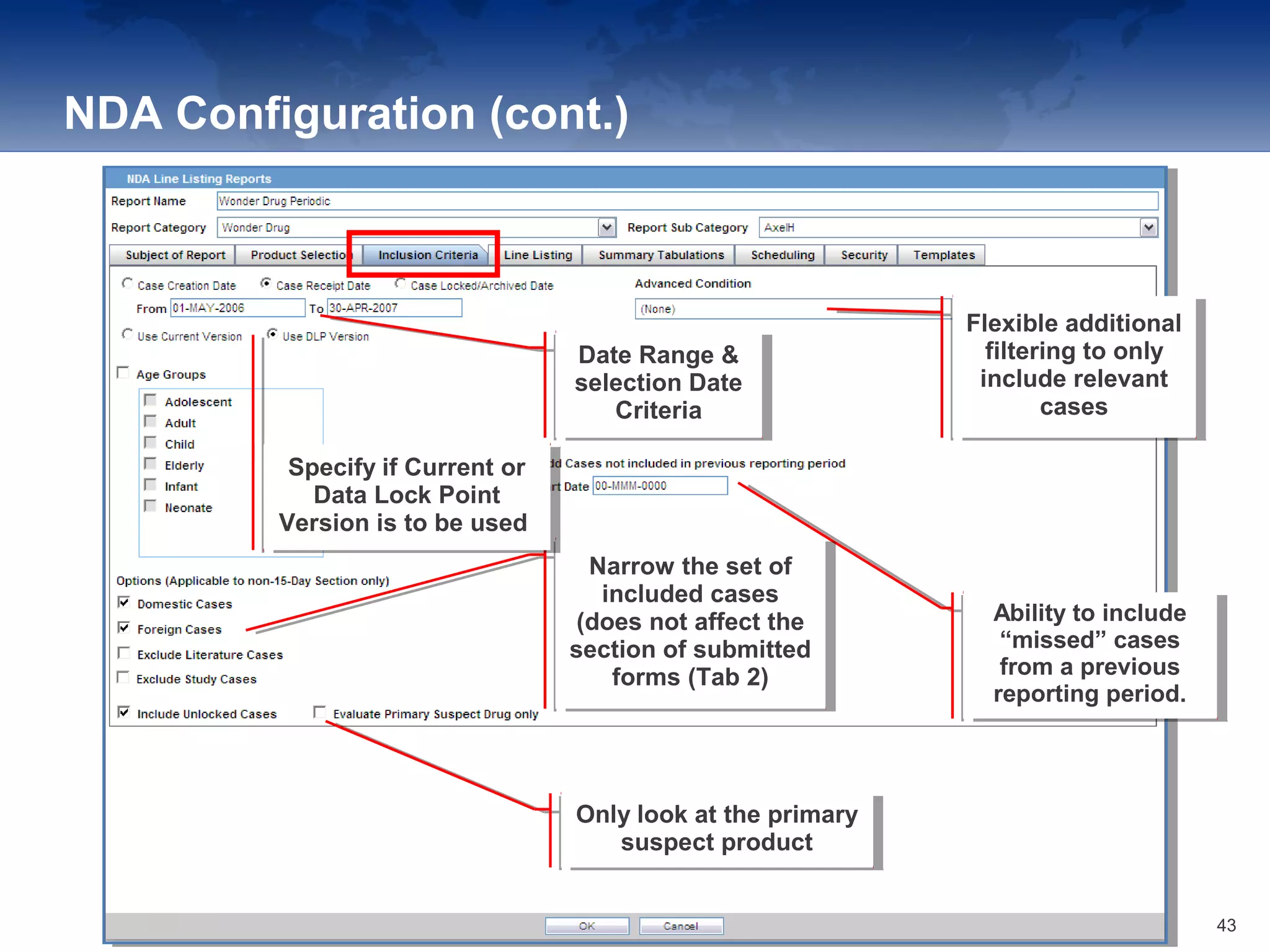
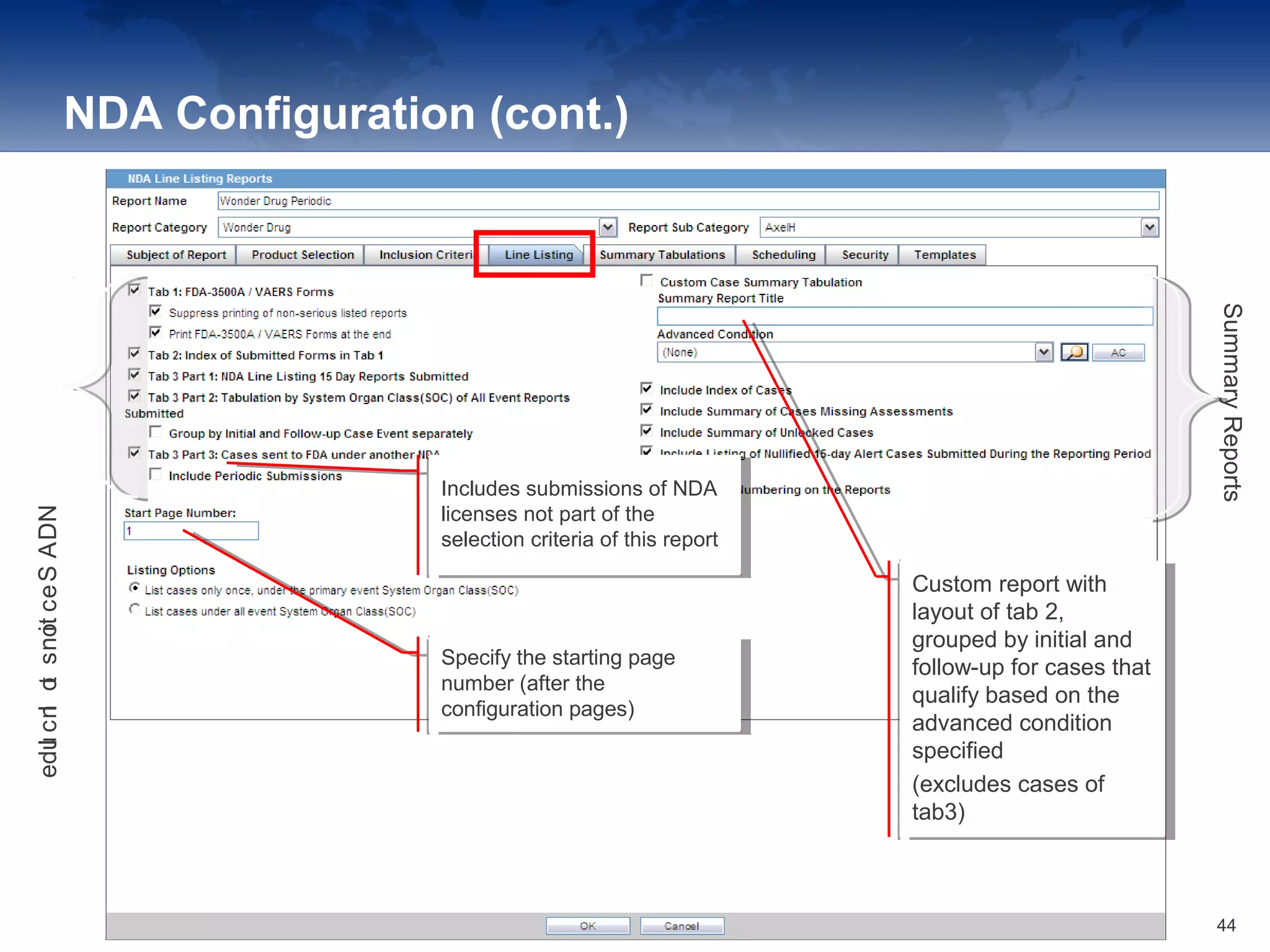
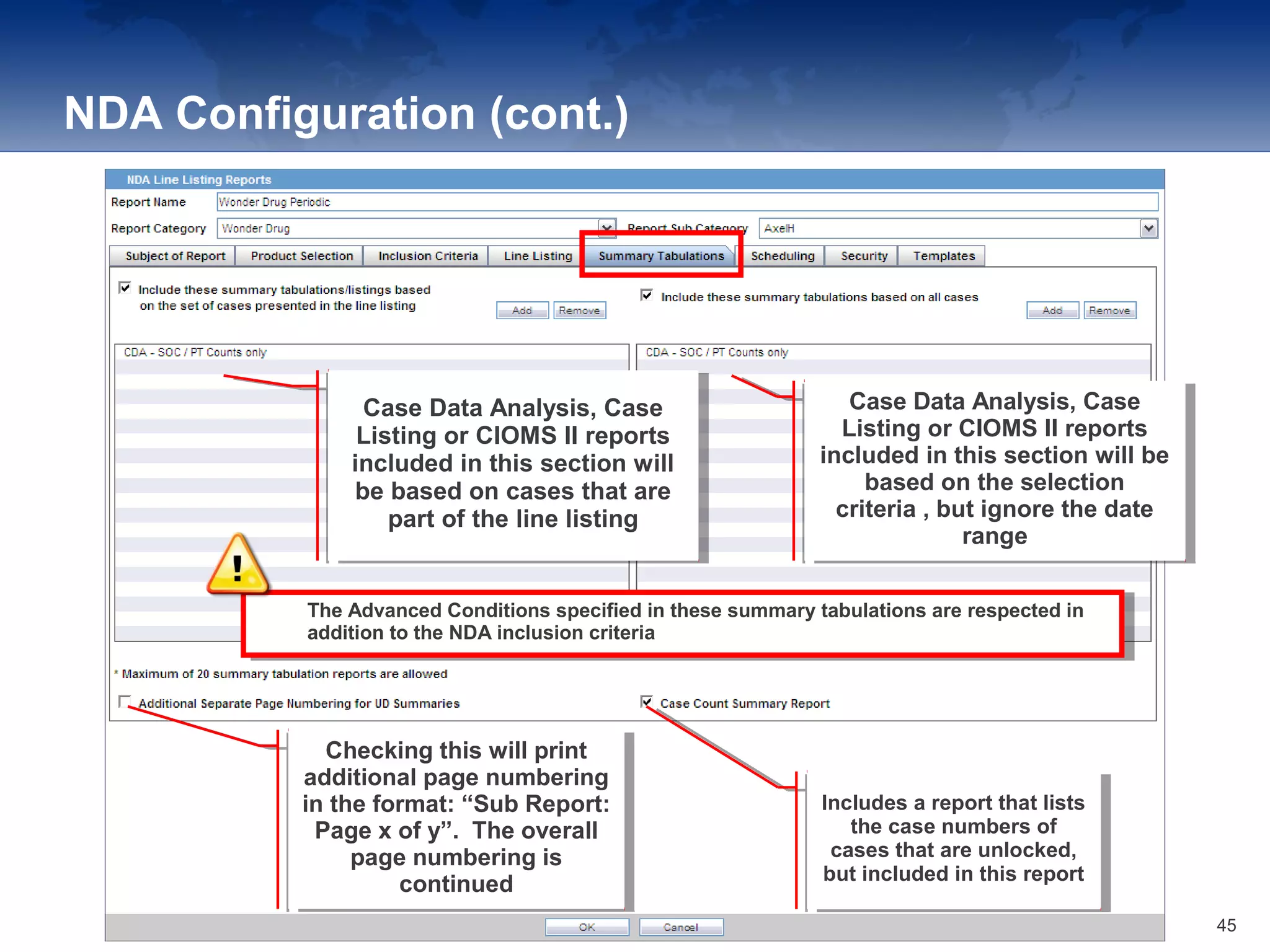
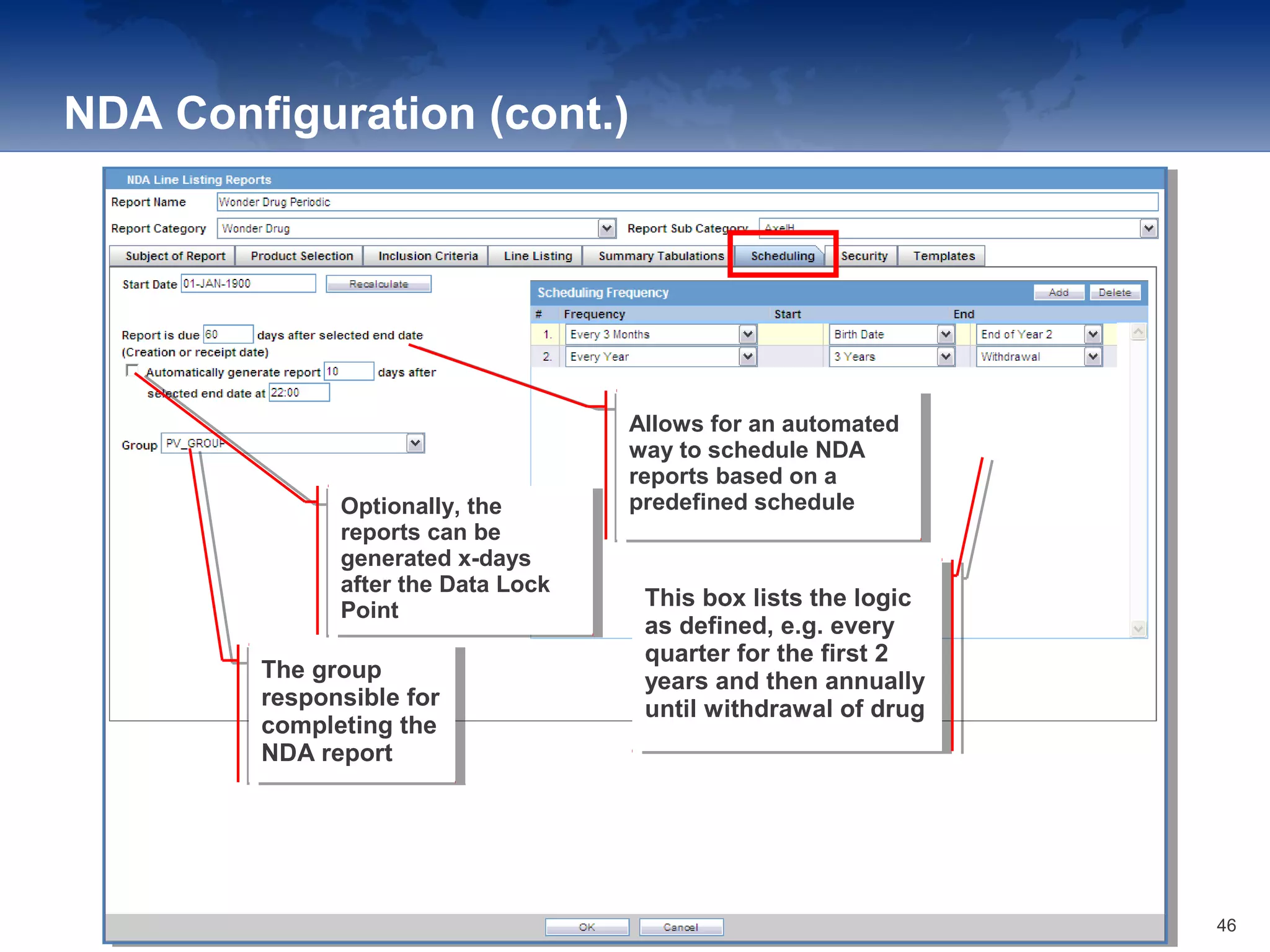
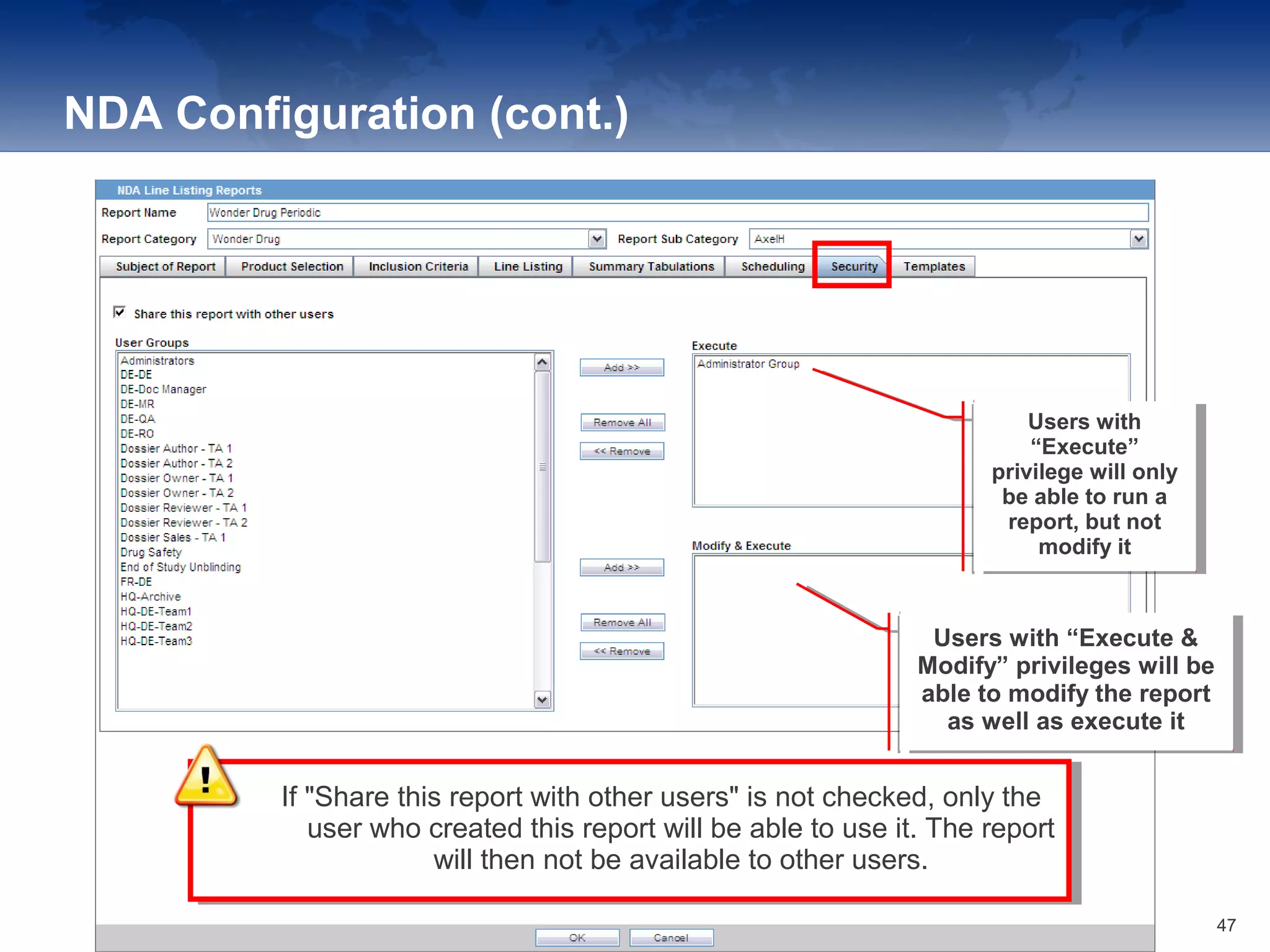
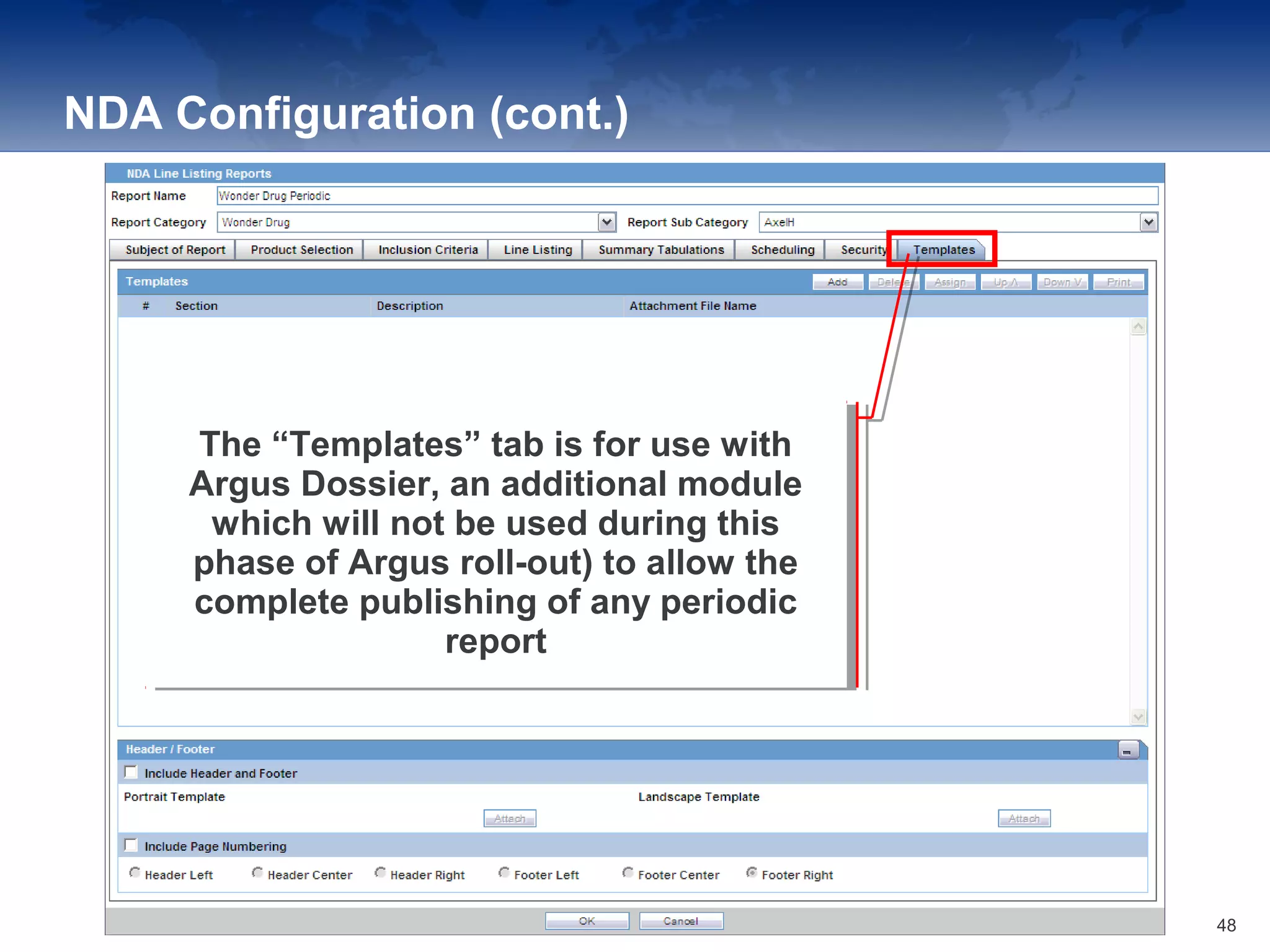
The document provides an overview of the reporting capabilities of Argus Safety, focusing on both aggregate and periodic reports. It details various types of reports that can be generated, such as case data analysis, CIOMS II line listings, and PSURs, along with instructions on accessing and configuring these reports. Additionally, it covers user privileges for report execution and modification, along with the importance of report scheduling and customization.