The document provides an overview of MedDRA, including:
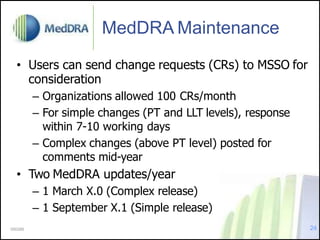
- MedDRA was developed under ICH to facilitate exchange of safety information. Its maintenance is overseen by an ICH committee.
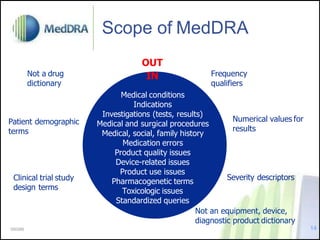
- It defines MedDRA as a medical terminology used for regulatory processes from pre- to post-marketing.
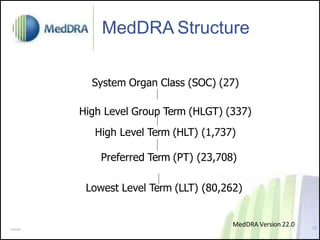
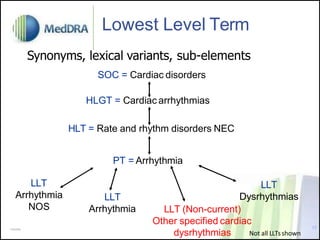
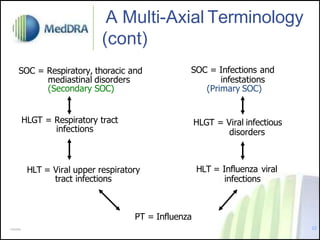
- MedDRA has a hierarchical structure of System Organ Classes, High Level Groups, High Level and Preferred Terms to classify adverse event information.