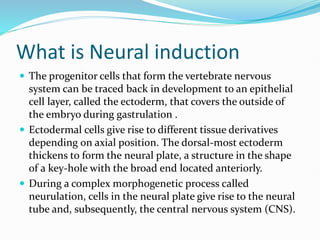
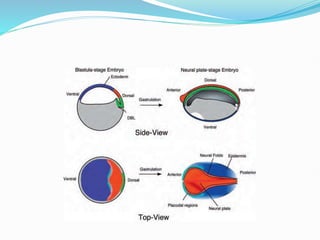
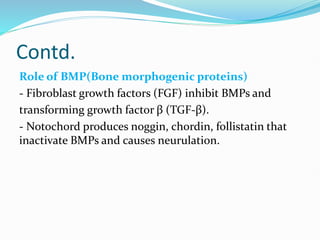
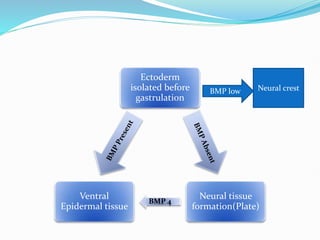
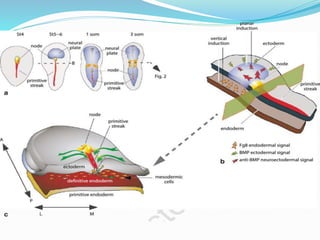
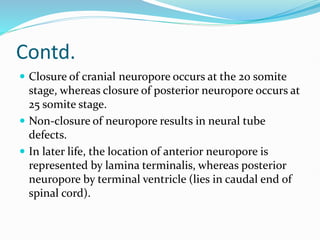
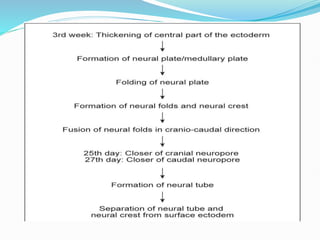
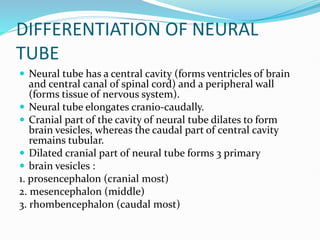
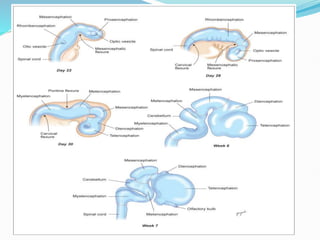
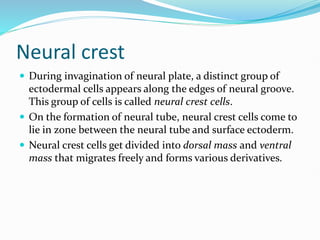
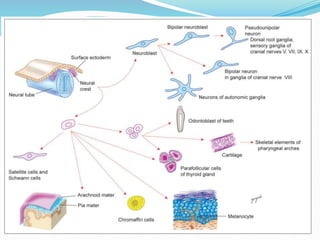
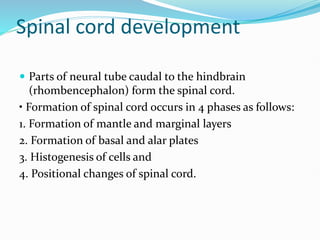
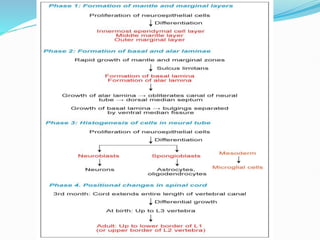
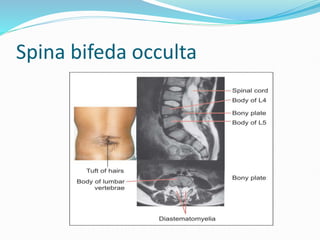
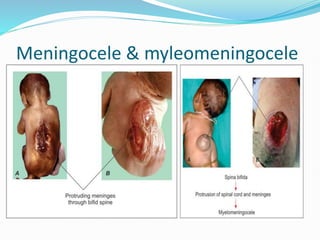
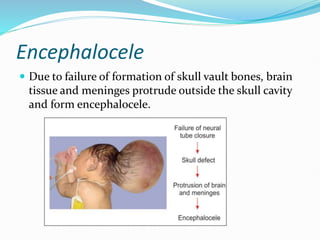
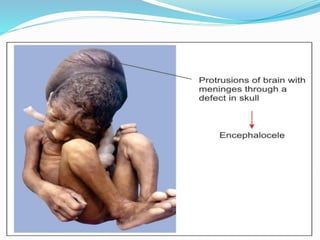
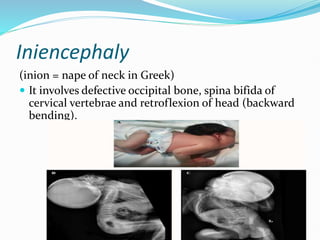
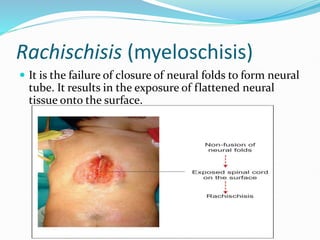
The document discusses neural induction and development of the nervous system from the ectoderm. It describes how the dorsal-most ectoderm thickens to form the neural plate during gastrulation. Through the process of neurulation, the neural plate folds in on itself to form the neural tube, which will later develop into the central nervous system. Neural crest cells emerge along the edges of the neural tube and go on to form many peripheral nervous system structures and other tissues. Failure of the neural tube to close properly can result in neural tube defects such as spina bifida.