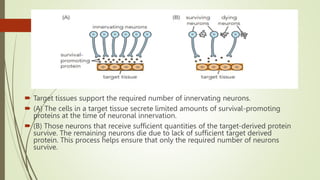
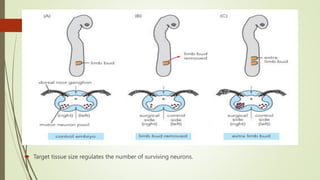
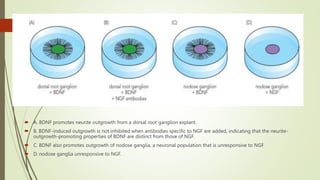
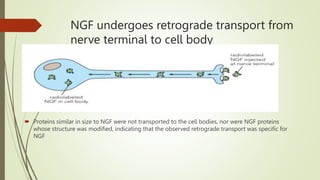
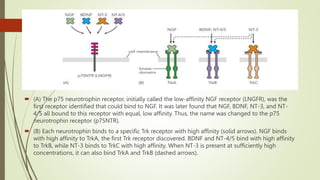
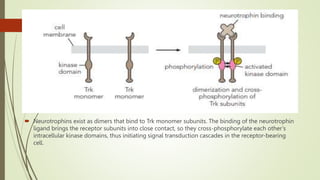
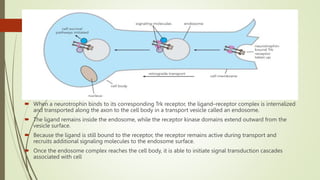
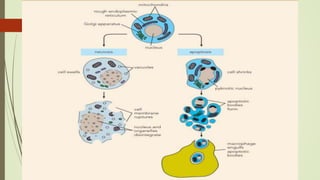
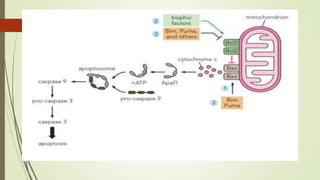
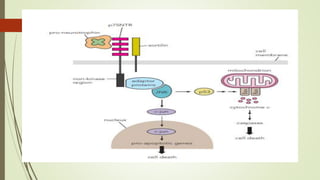
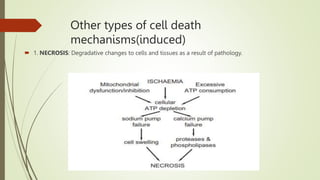
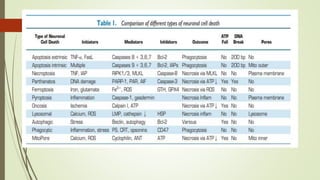
Target-derived neurotrophic factors ensure the correct number of neurons survive during development. Purified nerve growth factor promotes neuronal survival and prevents cell death. Major pathways of programmed cell death include the intrinsic mitochondrial pathway activated by loss of trophic factors, and the extrinsic pathway mediated by death receptors. Retrograde transport allows neurotrophins produced distant from the cell body to influence gene expression and survival.