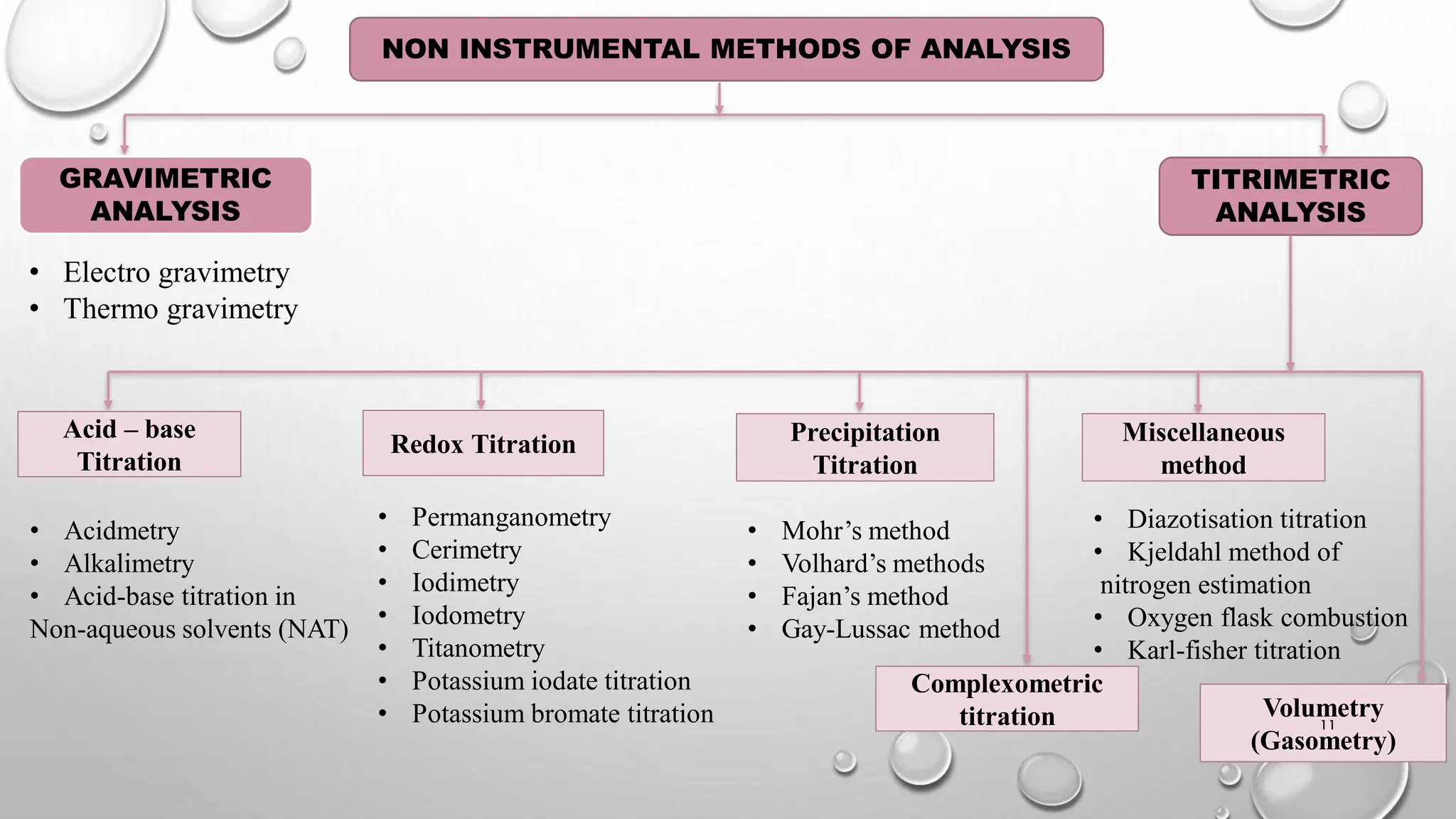
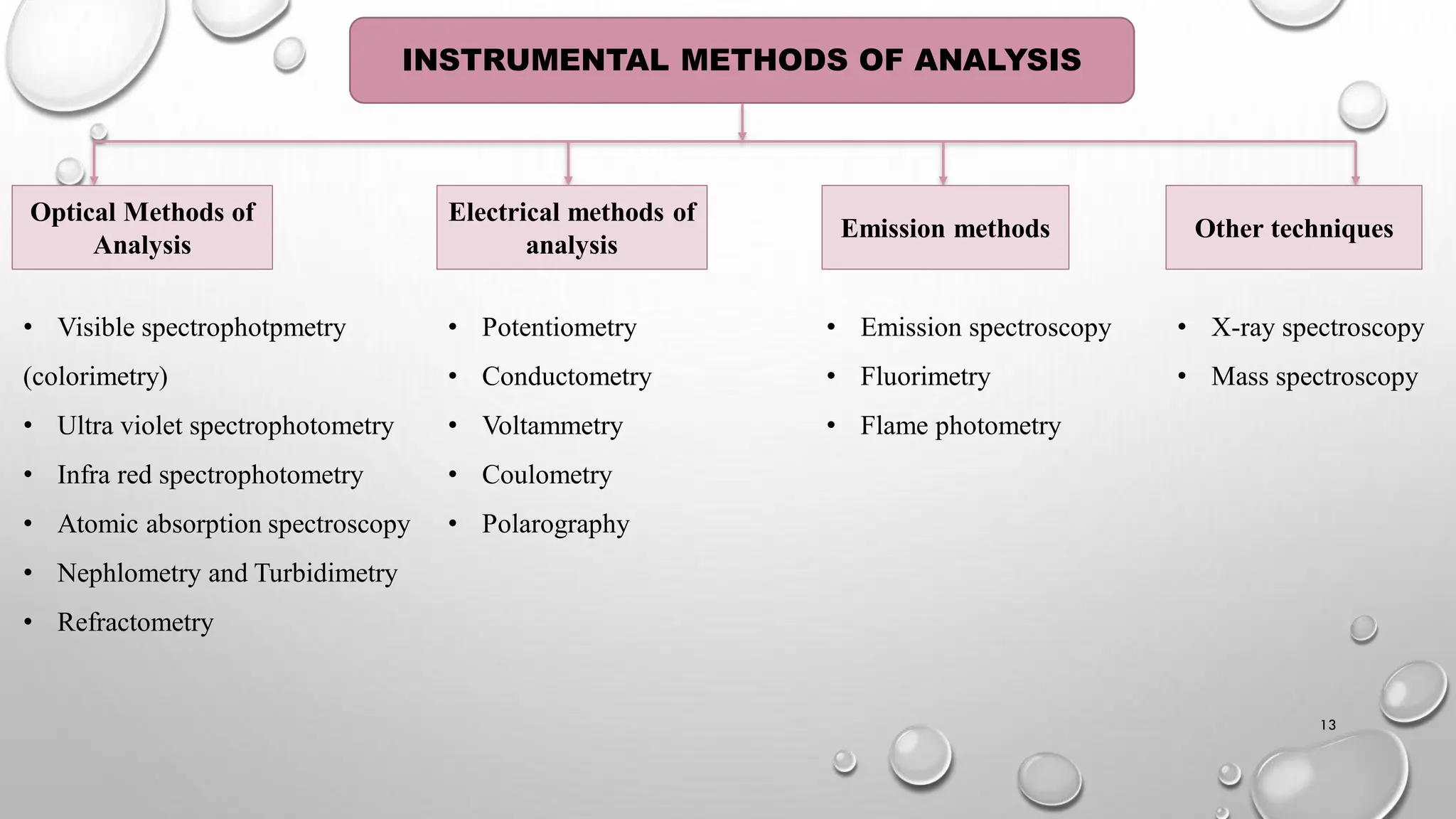
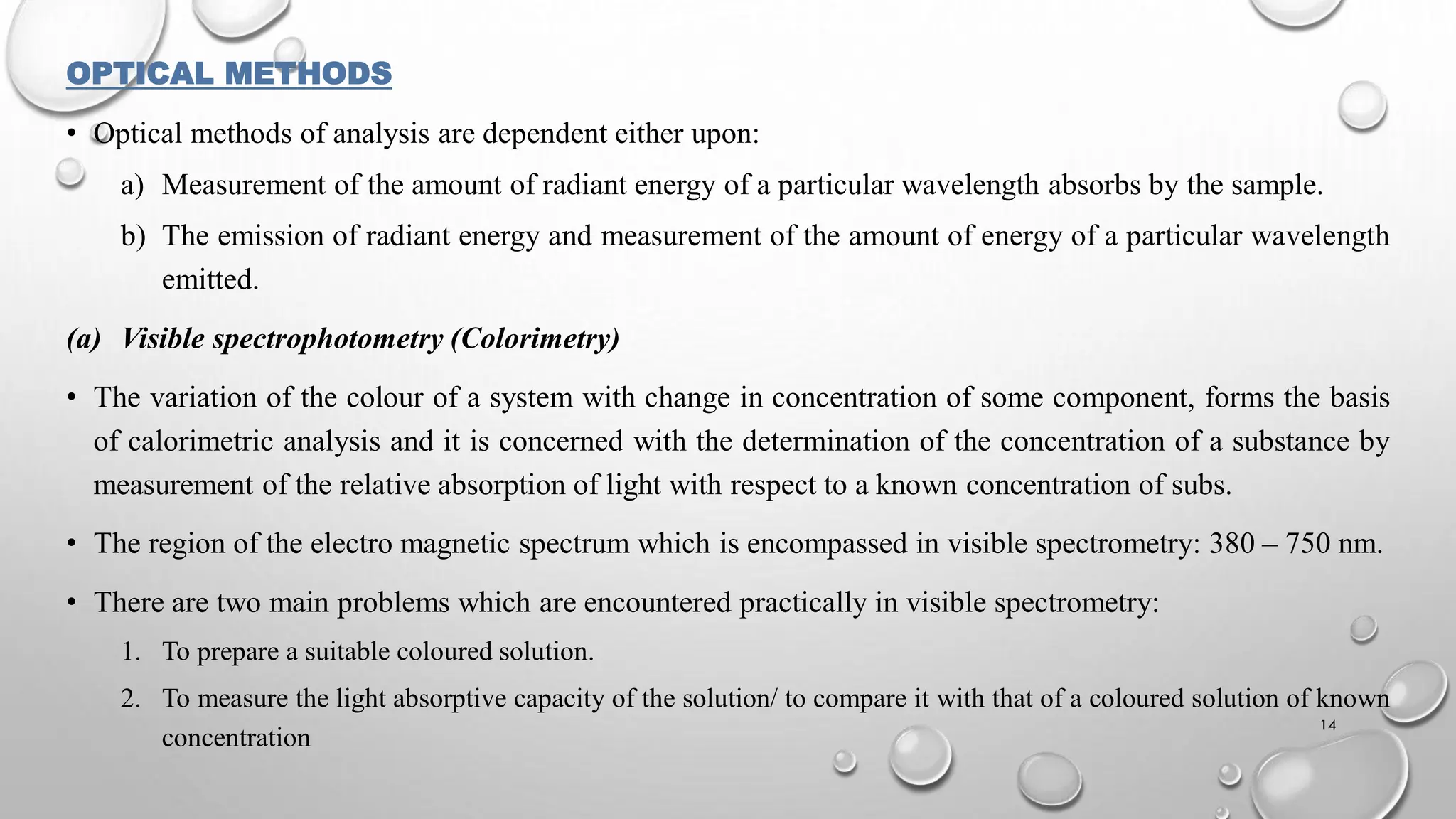
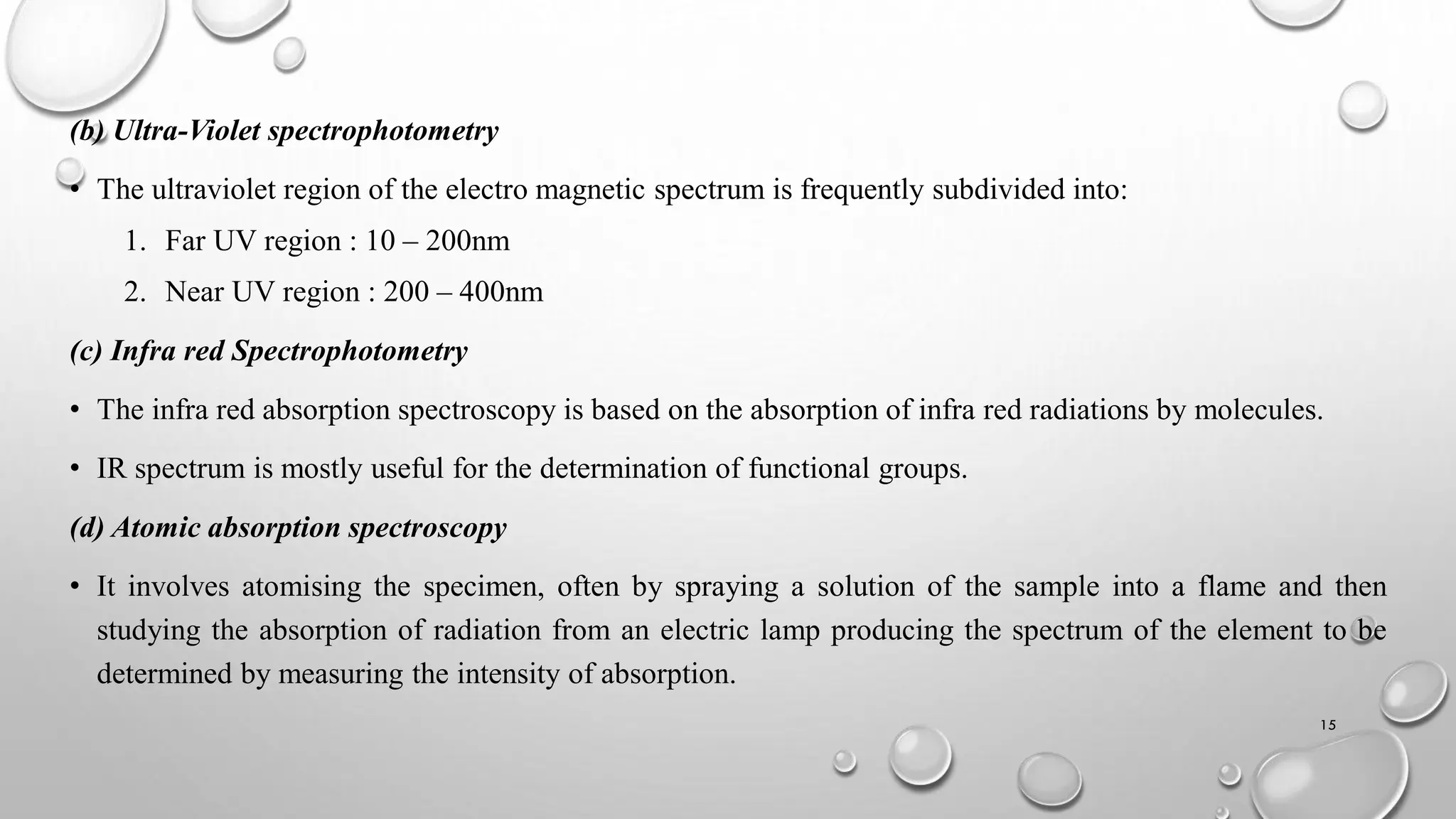
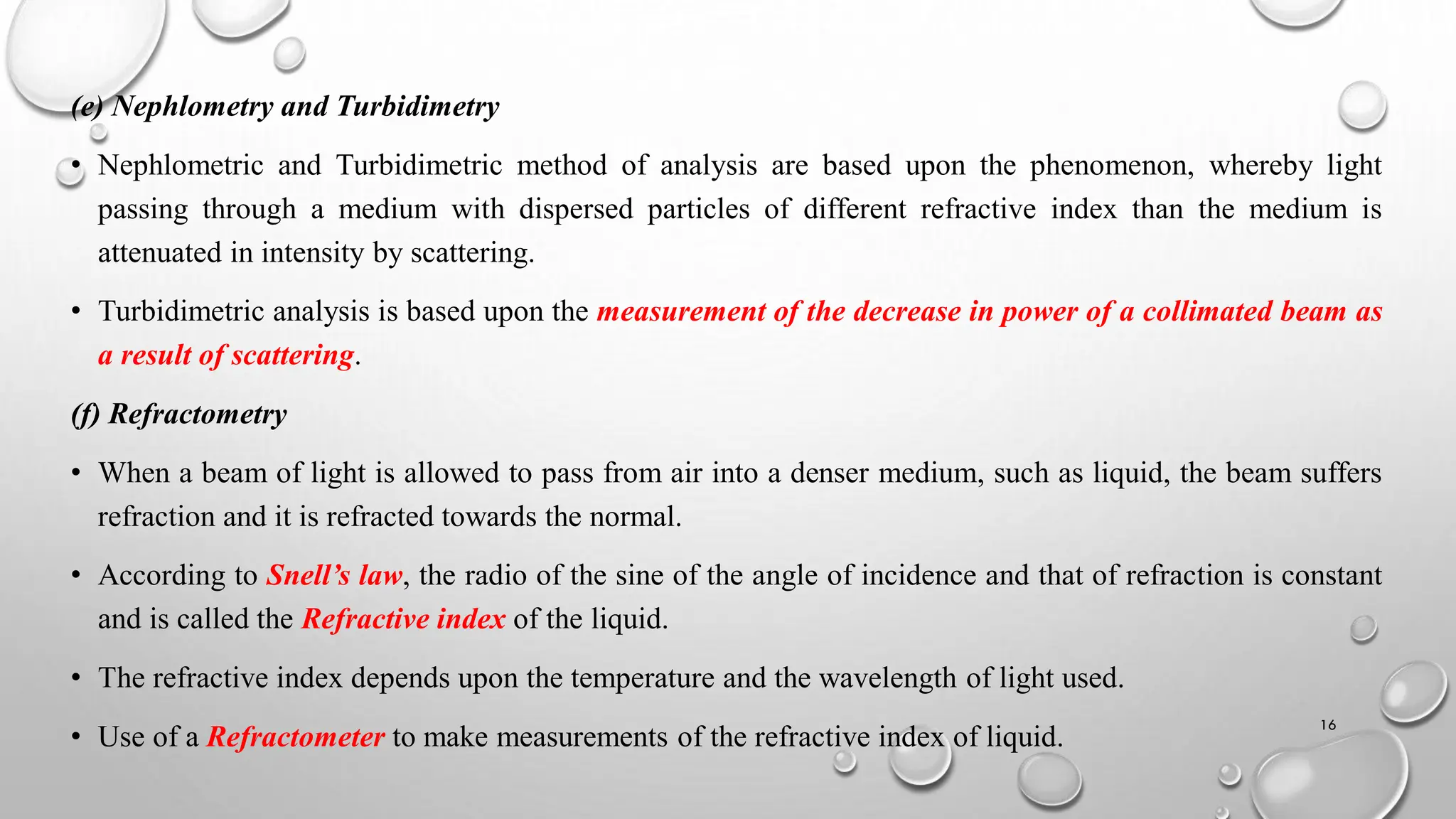
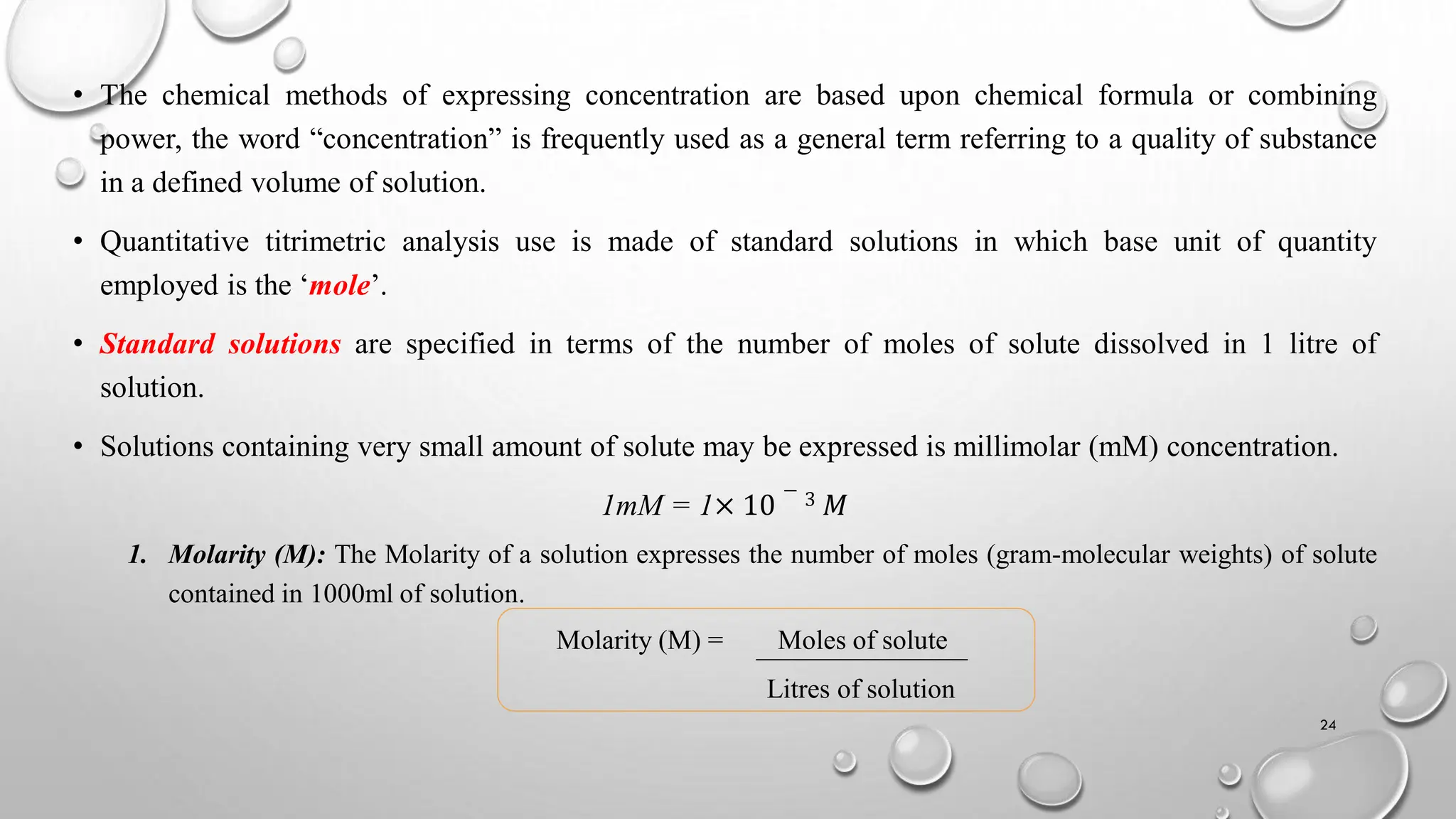
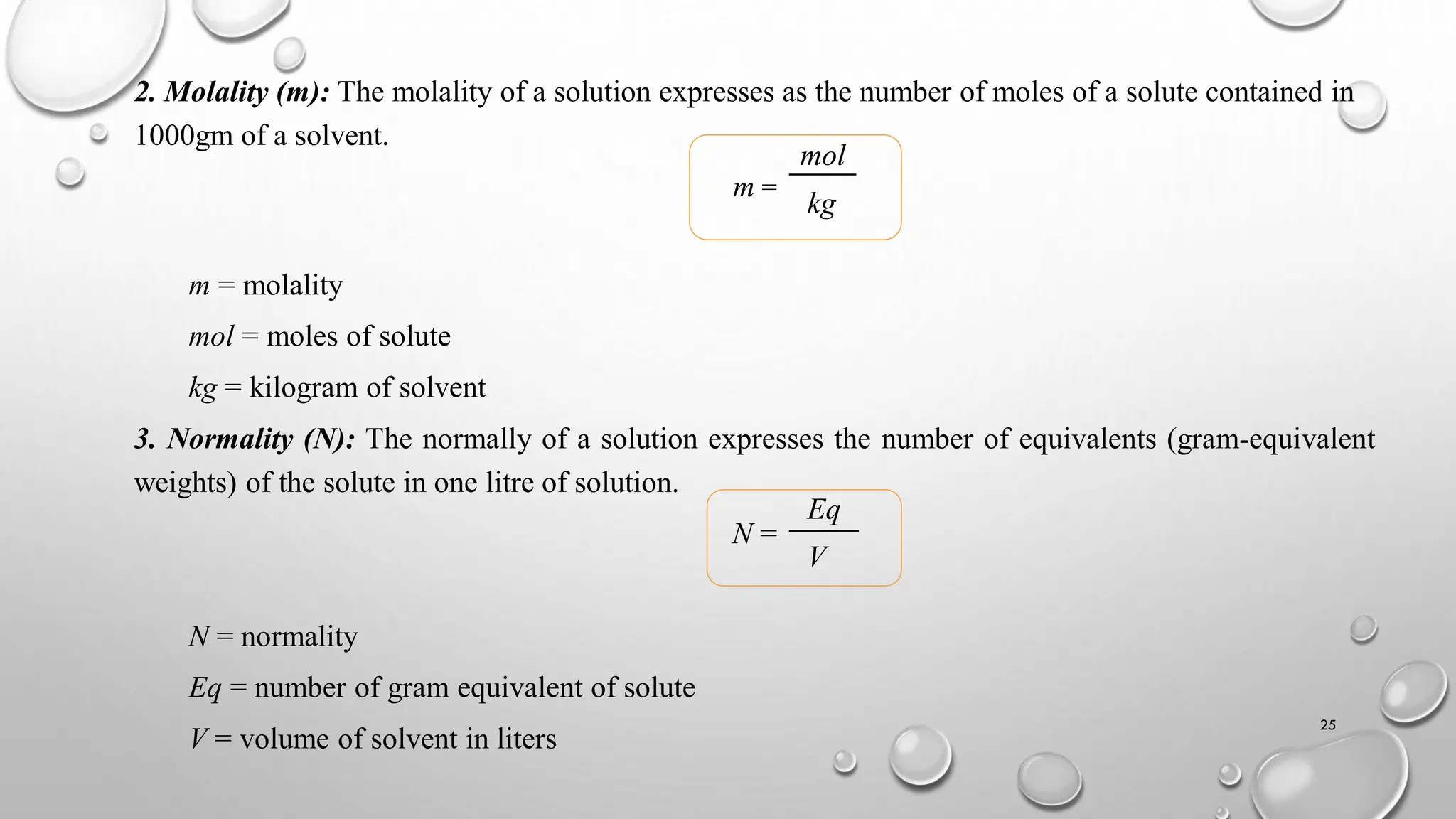
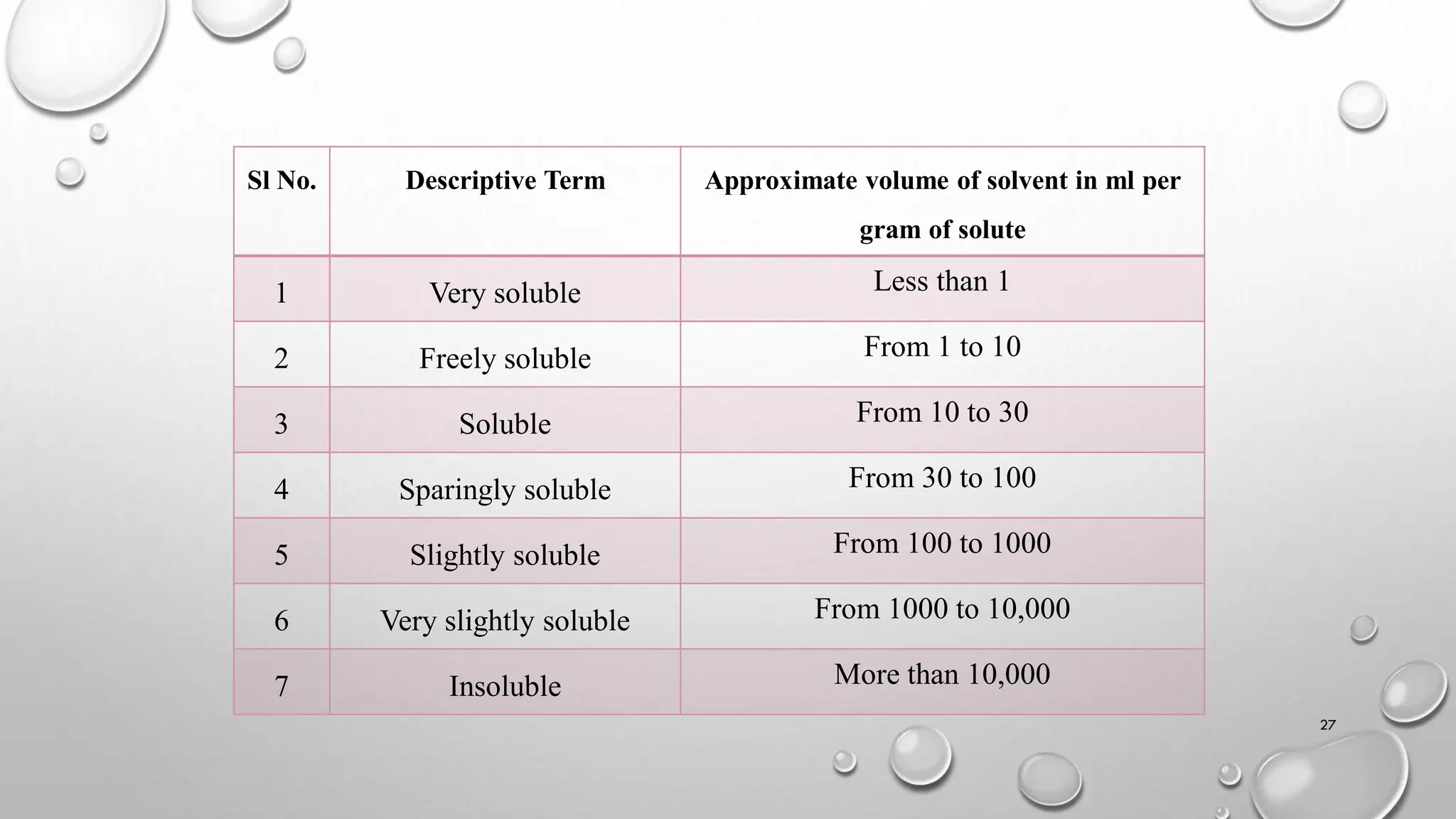
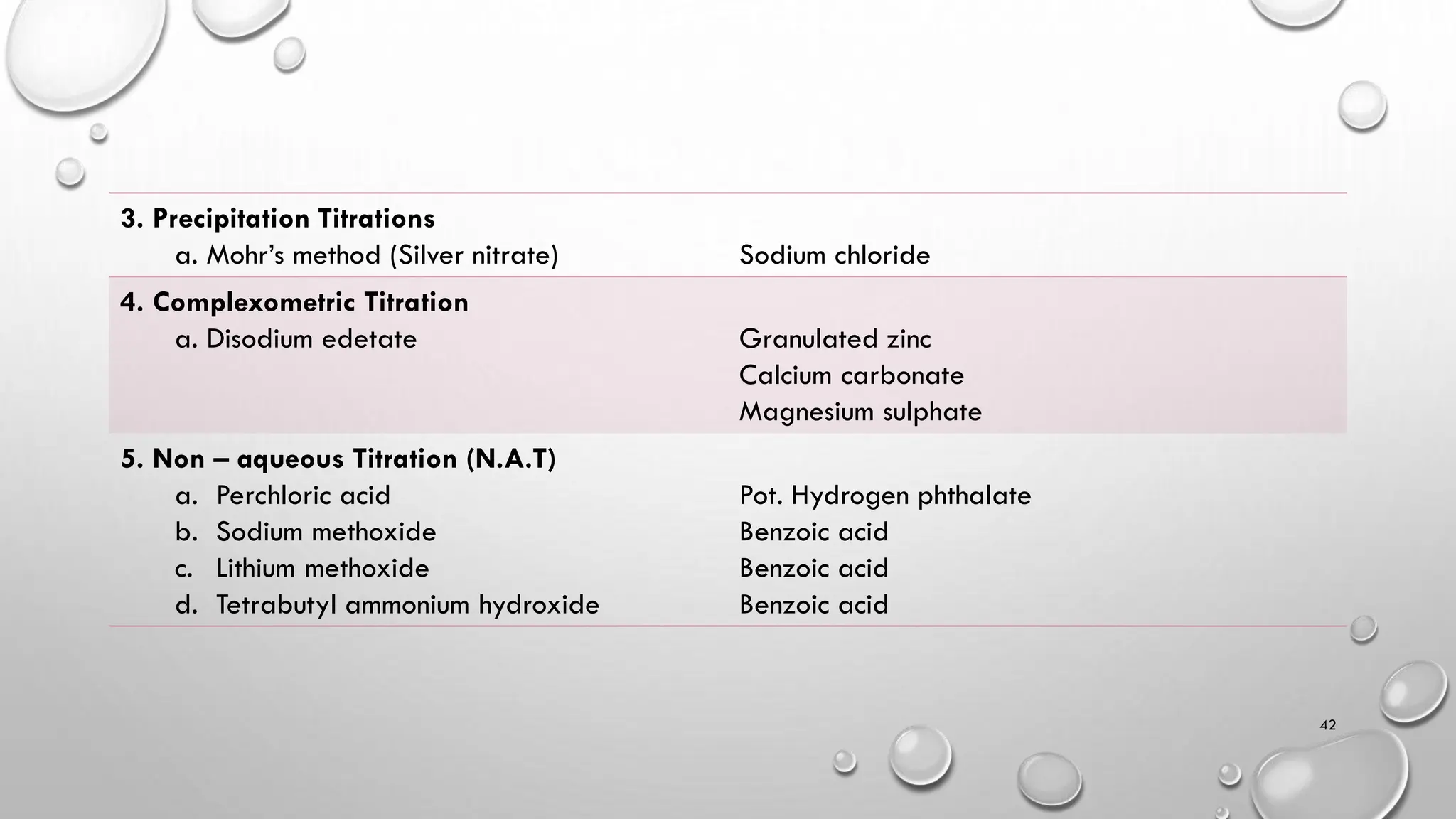
The document provides a comprehensive overview of volumetric analysis, detailing its types, techniques, and methods of expressing concentration. It distinguishes between qualitative and quantitative analysis, emphasizing the importance of identifying and estimating constituents in a sample. Additionally, the document covers different techniques of analysis, including non-instrumental and instrumental methods, and examines various ways to express concentrations in solutions.