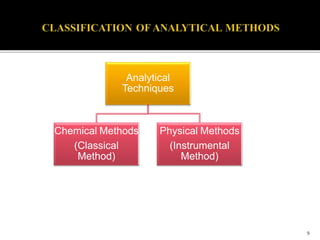
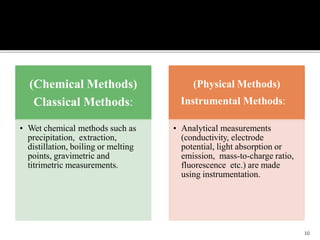
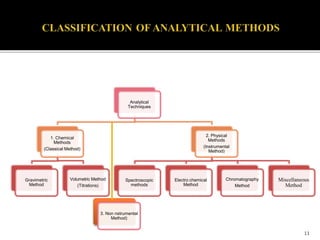
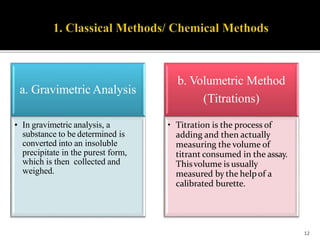
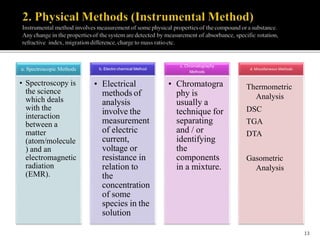
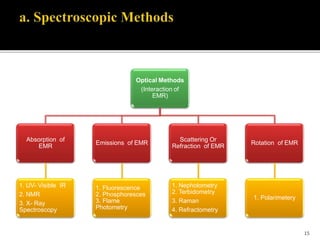
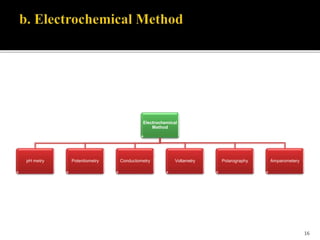
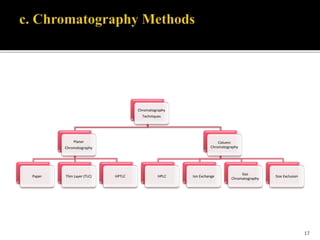
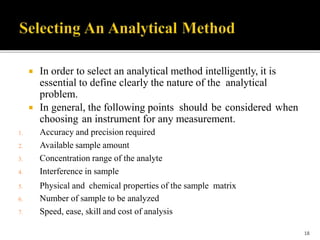
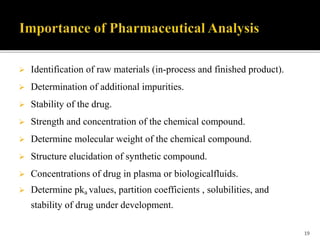
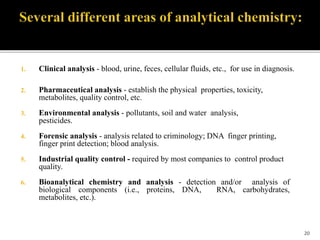
Pharmaceutical analysis involves analytical procedures to determine the purity, safety, and quality of drugs and chemicals. It is a branch of practical chemistry that identifies, quantifies, and purifies substances or separates mixtures. The objectives of the pharmaceutical analysis course are to understand principles of volumetric and electrochemical analysis and develop analytical skills. The course covers topics like sources of errors, primary and secondary standards, acid-base titrations, precipitation titrations, complexometric titration, and electrochemical methods of analysis. Pharmaceutical analysis is important for applications like quality control, clinical analysis, forensic analysis, and environmental analysis.