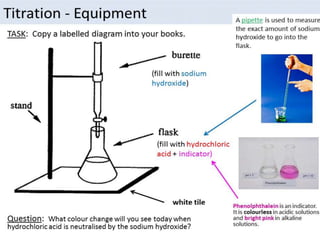
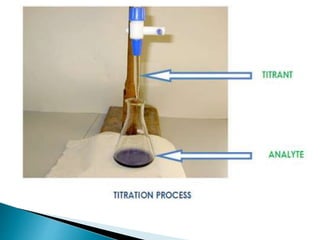
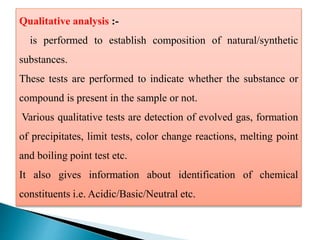
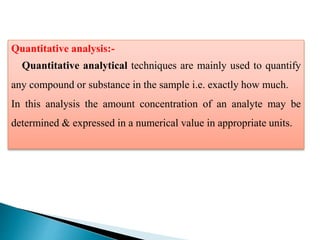
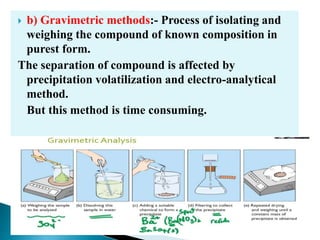
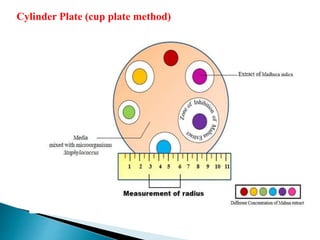
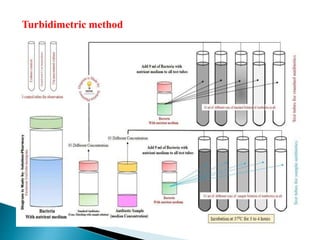
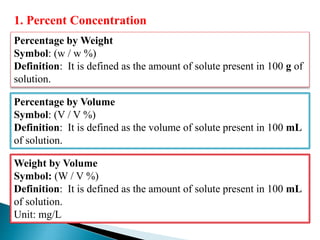
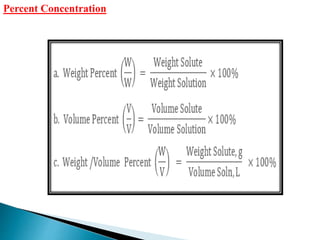
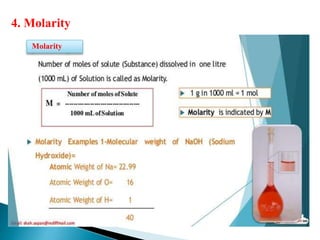
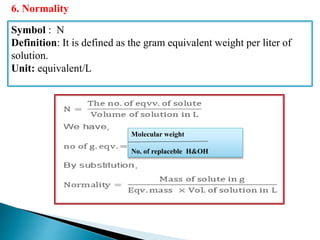
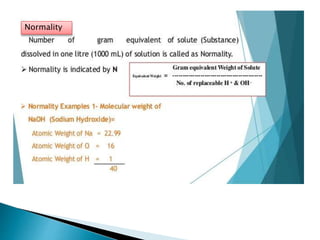
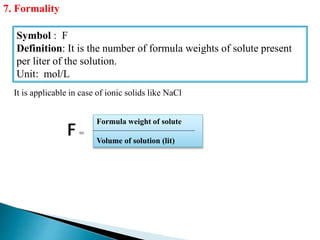
This document discusses various concepts related to pharmaceutical analysis including assay, analyte, titrant, titration, equivalence point, and standardization. It defines assay as a qualitative or quantitative assessment of an analyte using a titrant. Titration involves measuring the volume of titrant used to reach the equivalence point, as indicated by a color change from an indicator. The document also discusses different types of quantitative analysis techniques including volumetric (titrimetric), gravimetric, gasometric, and instrumental methods. It provides examples of concentration units used in pharmaceutical analysis such as molarity, molality, and normality.