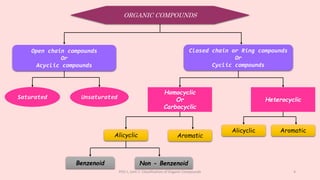
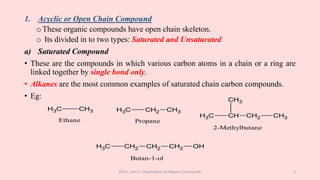
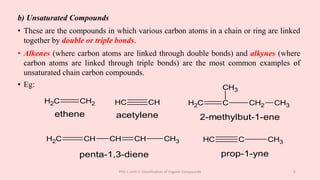
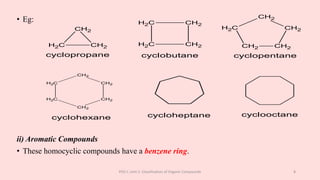
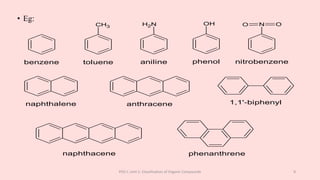
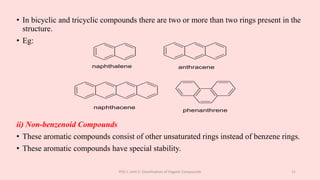
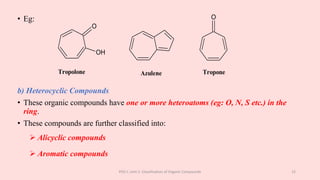
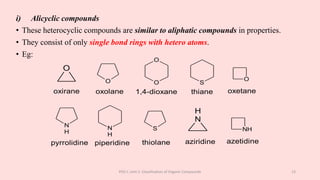
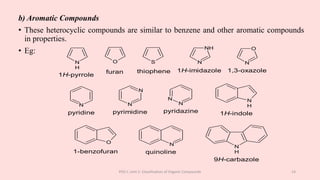
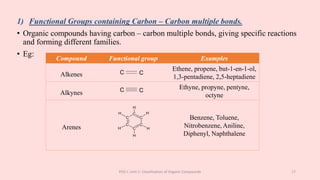
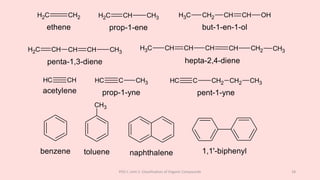
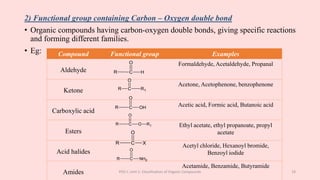
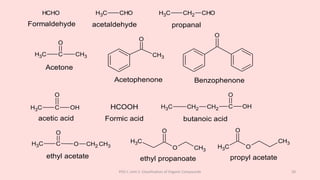
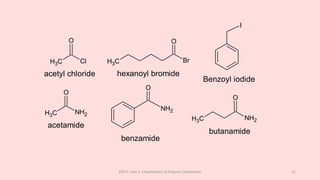
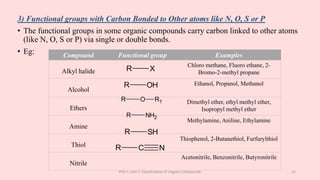
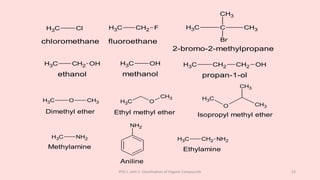
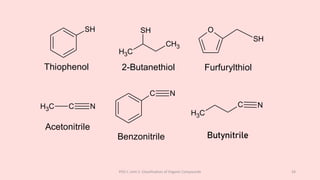
The document discusses the classification of organic compounds. Organic compounds can be classified based on their structure as open chain (acyclic) or closed chain (cyclic) compounds. They can also be classified based on functional groups. Cyclic compounds include homocyclic compounds containing only carbon and heterocyclic compounds containing heteroatoms like nitrogen. Functional groups include carbon-carbon multiple bonds, carbon-oxygen double bonds, and carbon bonded to other atoms. Common functional groups are alkenes, alkynes, aldehydes, ketones, alcohols, amines, and nitriles. Overall, the document provides an overview of how organic compounds can be systematically classified based on their structure and functional groups.