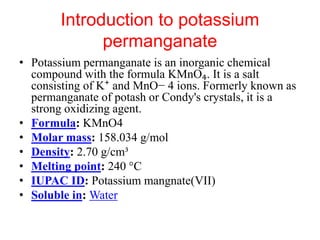
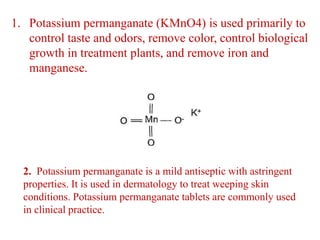
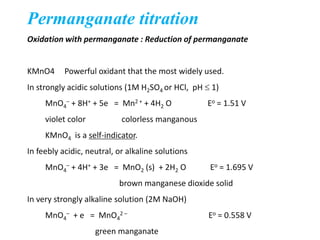
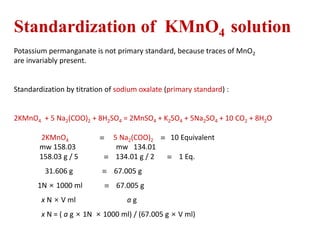
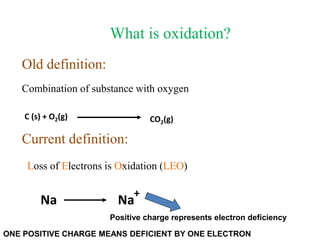
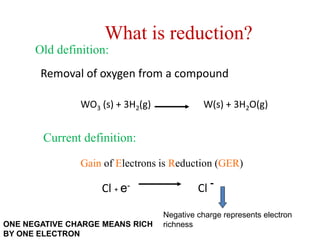
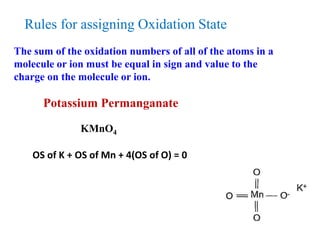
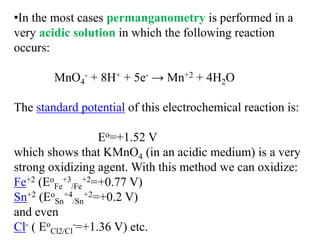
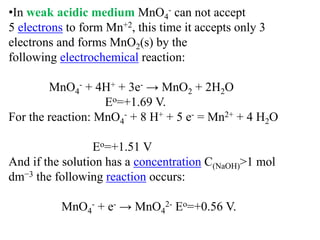
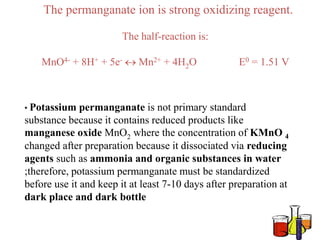
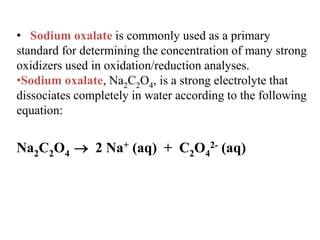
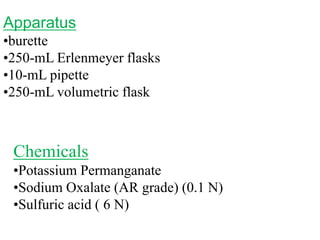
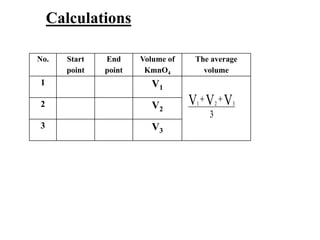
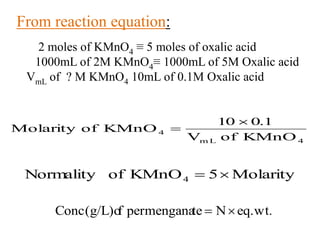
Potassium permanganate is being standardized by titrating it against a primary standard of sodium oxalate. Sodium oxalate is dissolved in sulfuric acid, and then titrated with potassium permanganate solution. The reaction causes the purple permanganate solution to become colorless. When the titration is complete, one extra drop of permanganate causes the solution to turn pink, indicating the endpoint of the reaction. The experiment is repeated three times and the average volume of permanganate used is calculated to determine its molarity.