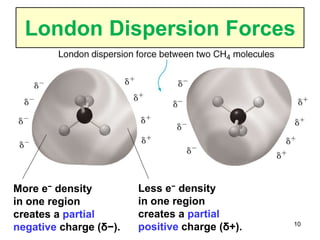
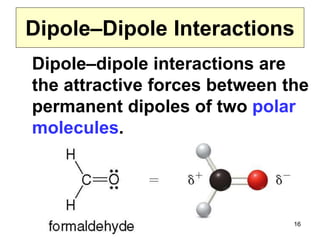
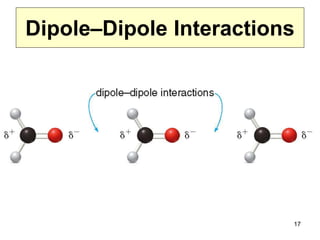
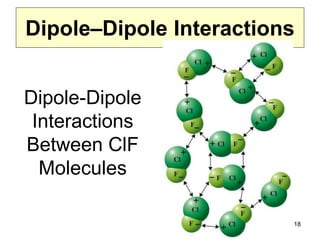
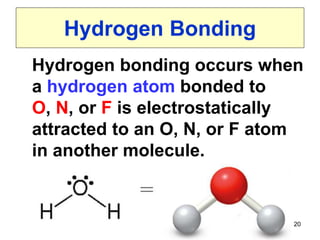
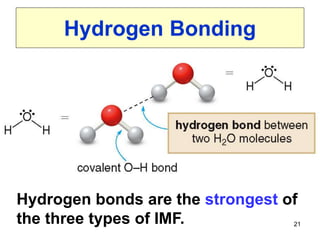
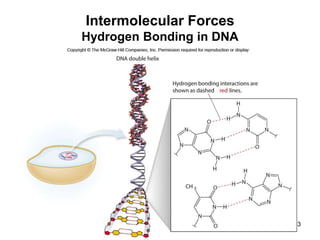
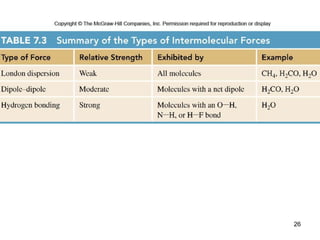
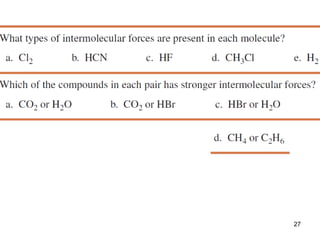
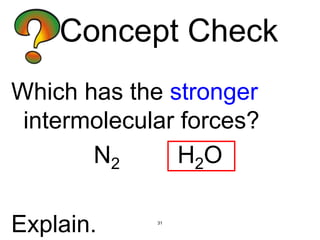
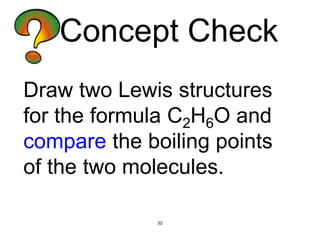
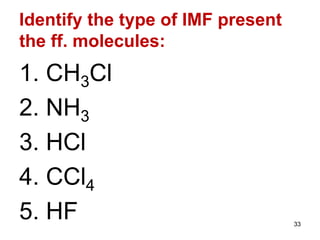
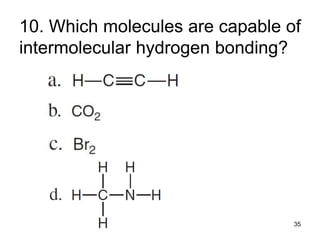
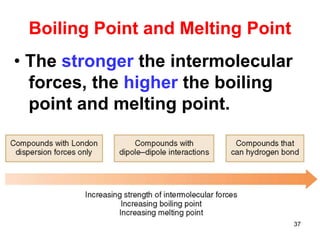
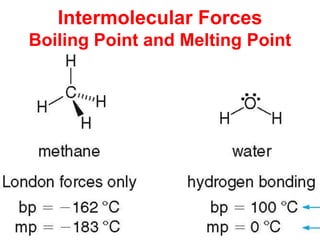
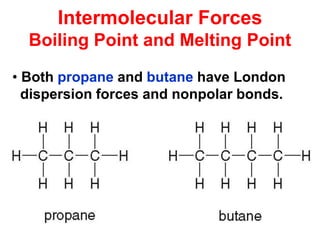
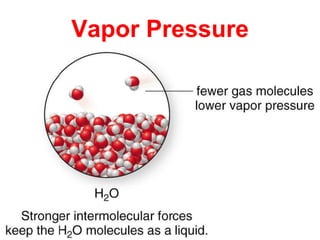
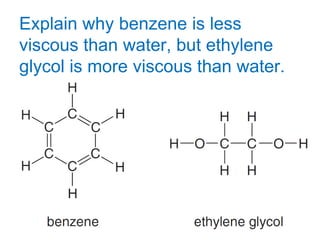
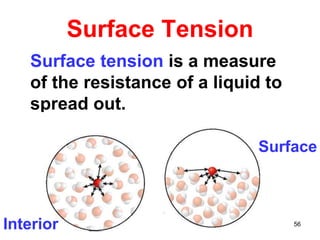
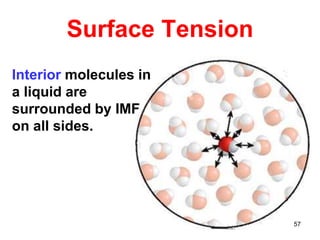
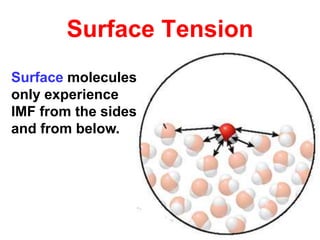
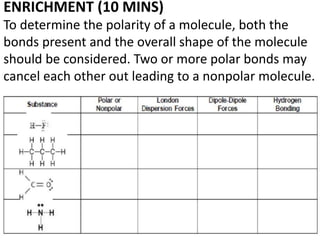
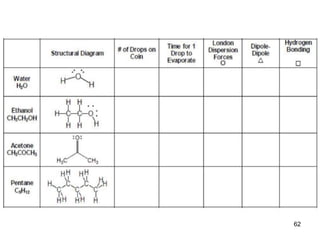
The document discusses different types of intermolecular forces including London dispersion forces, dipole-dipole interactions, and hydrogen bonding. It explains that intermolecular forces are weaker than intramolecular bonds but determine important physical properties like boiling point, melting point, vapor pressure, viscosity, and surface tension. Stronger intermolecular forces lead to higher boiling points and melting points, lower vapor pressure, and higher viscosity and surface tension.