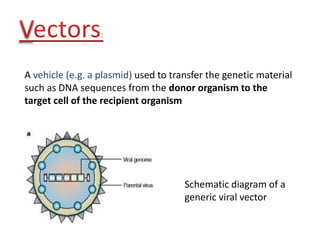
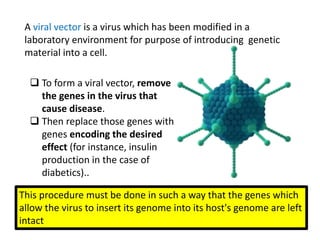
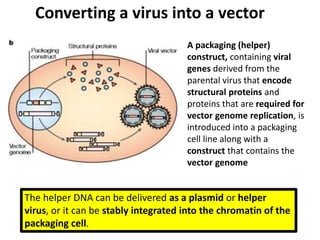
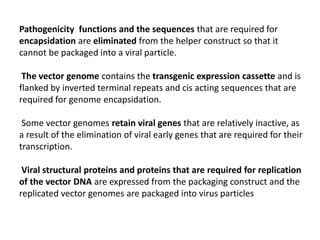
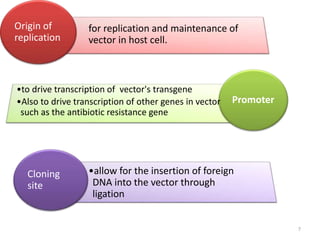
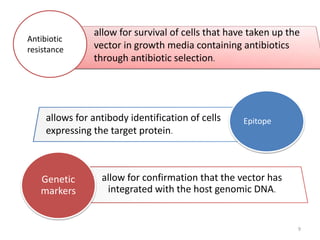
The document explains the concept of viral vectors as modified viruses used to transfer genetic material into cells for applications like gene therapy and vaccine production. Various types of viral vectors are described, including DNA and RNA-based vectors, as well as their functions, safety considerations, and design characteristics. The document highlights the importance of ensuring low toxicity and stability while achieving specificity in targeting cells, as well as the role of viral vectors in cancer treatment and immunology.