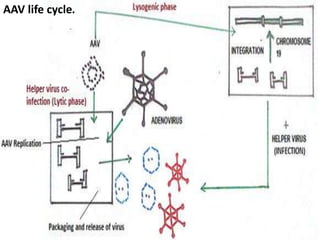
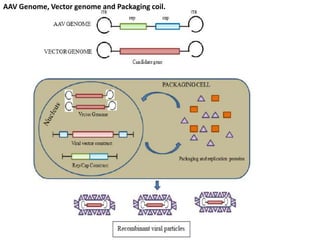
Adeno-associated virus (AAV) is a small, non-enveloped virus that was first discovered in 1965 as a contaminant in adenovirus isolates. It requires a helper virus like adenovirus or herpes virus to productively infect cells. AAV has a life cycle with both lytic and lysogenic phases - in the presence of a helper virus it undergoes lytic replication and production of new virions, while in the absence of helper virus it can integrate into the host genome and remain latent. Recombinant AAV (rAAV) vectors are used in gene therapy, containing a transgene in place of the viral genes, with only the inverted terminal repeats remaining to function in