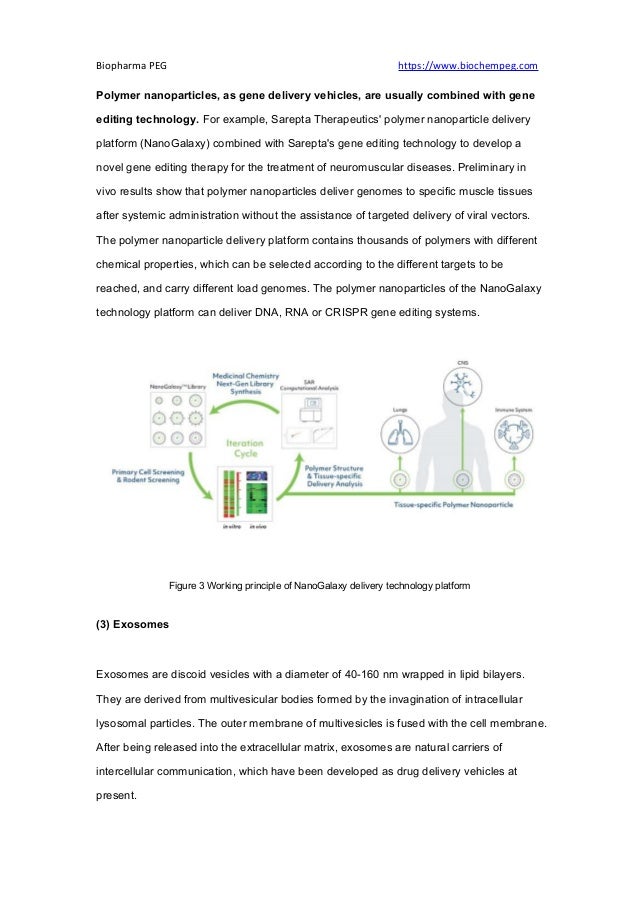
The document discusses advancements in gene therapy, highlighting its significance as a method for correcting defective genes to treat diseases, in contrast to traditional chemotherapy. It categorizes gene therapy vectors into viral (e.g., lentivirus, adenovirus, adeno-associated virus) and non-viral (e.g., lipid nanoparticles, polymer nanoparticles, exosomes) vectors, noting that viral vectors dominate clinical trials but face challenges like production and safety. Non-viral vectors are emerging due to their advantages, including lower toxicity and ease of production, making them a promising alternative for future gene therapy applications.







![Biopharma PEG https://www.biochempeg.com
160743-62-4
3 mPEG-CH2CH2CH2-NH2 ---
4 mPEG-OH
CAS NO.:
9004-74-4
5 mPEG-CM (mPEG-AA) ---
6 mPEG-DSPE
CAS NO.:
147867-65-0
7 mPEG-DPPE
CAS NO.:
205494-72-0
References:
[1] Lentiviral Vector Pseudotypes: Precious Tools to Improve Gene Modification of Hematopoietic Cells
for Research and Gene Therapy.
[2] Adenovirus: the first effective in vivo gene delivery vector.
[3] AAV-Mediated Gene Therapy for Research and Therapeutic Purposes.](https://image.slidesharecdn.com/overviewandvectorsforgenetherapy-220309022645/95/Overview-and-vectors-for-gene-therapy-9-638.jpg)