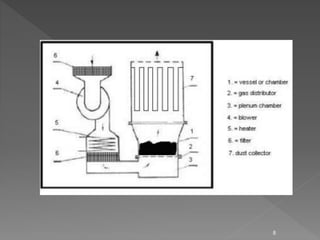
The document discusses validation and qualification protocols for a fluidized bed dryer (FBD). It describes the construction and working of the FBD, including fluidization principles. Validation includes design, installation, operational, and performance qualification steps to ensure the FBD operates as intended. Qualification tests are conducted to demonstrate that the FBD can consistently dry materials as specified and produce products meeting quality standards.