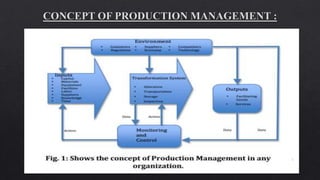
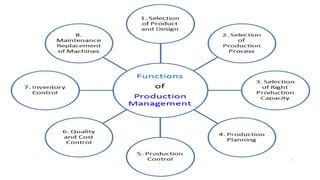
1. The document outlines requirements for modern pharmaceutical production facilities including location, building design, waste disposal, storage, production, and quality control. Key requirements include preventing contamination, ensuring hygienic conditions, and separating different categories of drugs.
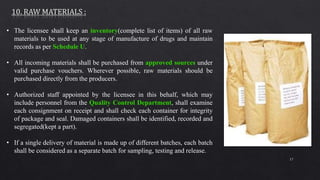
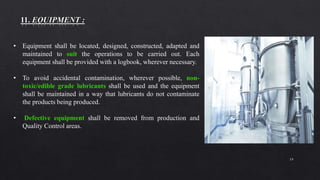
2. Specific sections cover water treatment systems, warehousing, production area layout, quality control laboratory independence, personnel training, and inventory/raw material management. Equipment must be properly located, maintained and defective units removed.
3. Quality assurance systems must ensure pharmaceutical products meet GMP standards and are developed to requirements for design, development, manufacturing and quality management.