This document discusses fluidized bed dryers (FBDs) used in pharmaceutical manufacturing. It provides information on:
1) The principle of fluidization where hot air is passed through granules in a container, lifting and suspending them in a "fluidized state" for drying.
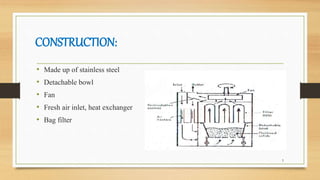
2) The construction of FBDs using stainless steel with a detachable bowl, fan, filters and air inlets/outlets.
3) The working where granules are placed in the dryer and hot air flows through them to achieve drying before the air exits.